|
Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice
長期採用生酮飲食可能造成糖尿! 2018年蘇黎世兒童醫院研究,給小老鼠餵食生酮飲食,結束後反而造成血糖不穩定和震盪等現象。研究發現,長時間生酮的老鼠,胰腺細胞也用盡廢退,萎縮掉了。 Mechanisms of starvation diabetes: a study with double tracer and indirect calorimetry pubmed.ncbi.nlm.nih.gov/2260646/
0 評論
阿茲海默症(Alzheimer’s disease)治療出現突破性進展!美國時間3日,禮來(Eli Lilly)宣布其開發的 抗體新藥donanemab,在臨床三期試驗中顯著減緩早期阿茲海默症患者的認知與功能性退化。對 醫學界來說,以類澱粉蛋白(amyloid)為標靶的donanemab展現出積極功效,也為「清除類澱粉蛋 白」與「減緩認知功能退化」的理論提供了更有力的支持。
在這項名為TRAILBLAZER-ALZ 2的臨床三期試驗中,招募了超過1700名受試者,招募對象為出現輕 度認知受損(MCI)以及輕度失智症狀,並確定出現神經病理學特徵的早期阿茲海默症患者。受試者 會在達成特定標準的類澱粉斑塊沉積清除後,才算是完成治療流程。 結果顯示,經過綜合評估量表Clinical Dementia Rating-Sum of Boxes (CDR-SB)評估, donanemab組患者在1年後有將近一半(47%)的患者未出現臨床惡化,安慰劑組則是29%。 在減緩臨床退化方面,donanemab組則是35%,在執行日常生活項目的能力上,退化程度也減緩 了40%;此外,超過一半的患者,都在投藥第12個月完成了他們的治療流程。 禮來也在這項研究發現,比起腦中tau蛋白濃度偏高的患者,tau蛋白濃度居中的患者治療效果更 好。布萊根婦女醫院阿茲海默症研究及治療中心主任Reisa Sperling認為,盡可能在早期階段就移 除類澱粉蛋白,可能是一種大幅減緩患者退化的方式。 撰文 日期 禮來表示,他們預計在6月底前為donanemab申請完全批准(full approval),如此美國食品藥物管 理局(FDA)可能就會在今年稍晚或2024年初做出批准決定。 外媒認為,donanemab將能與百健(Biogen)/衛采(Eisai)開發、甫於今年1月獲得FDA加速批准 (accelerated approval)的Leqembi (lecanemab)一較高下,且可能更有競爭力,因為Leqembi在 CDR-SB的評估中,減緩認知退化的幅度為27%。 不過,醫學專家則認為,現在要比較兩種藥物的效果還言之過早,但兩者在投藥頻率方面倒是有重 大差異:Leqembi是每2週注射一次、且沒有結束時間,但donanemab是每4週注射一次,且只要 持續到腦部掃描類澱粉蛋白檢測結果呈現陰性即可,在療程上更方便。 另一方面,針對此次禮來公布的成果,外媒仍指出,雖然大多數的患者都受益,但在該試驗中,還 是發生了三起可能與該藥物相關的死亡;此外,donanemab的安全性與有效性和Leqembi如何比 較,也是他們關心的重點。禮來則表示,他們將會在今年7月荷蘭阿姆斯特丹舉辦的醫學會議中, 透露更多研究細節。 《Cell》子刊:脂肪肝促大腸直腸癌肝轉移! 科學家籲勿輕忽風險 (圖片來源:網路)
近(11)日,美國錫安山醫學中心(Cedars-Sinai Medical Center)的科學家,發現了一項脂肪肝可促進大腸直腸癌(CRC)轉移的新機制脂肪肝細胞會透過分泌「促進腫瘤細胞向肝臟擴散」的蛋白質和遺傳物質囊泡;且即使只是輕度的脂肪肝,仍會增加腫瘤擴散的風險。該研究發表於期刊《Cell Metabolism》。 研究團隊表示,目前已知肝轉是移造成大腸直腸癌死亡的主因之一,且脂肪肝會促進肝轉移發生,不過其中的機制仍未知。 針對此進行研究後,他們發現,脂肪肝患者的肝細胞所分泌的細胞外囊泡(EV),會透過促進腫瘤生長「Yes相關蛋白」(Yes-associated protein)訊號,及讓免疫微環境變得有利於腫瘤,進而促使大腸直腸癌的肝轉移。 研究人員發現,脂肪肝的肝臟細胞會經由增加胞外體釋放因子Rab27a的表現,促進細胞外囊泡產生,這些囊泡內含三種會刺激癌細胞增殖、遷移,並增加侵略性的小分子核糖核酸(microRNA),當癌細胞吸收這些囊泡後,這些microRNA會和Yes相關蛋白發生反應,進一步促進轉移的腫瘤細胞生長。 另外,Yes相關蛋白還會抑制腫瘤微環境中的免疫系統,研究人員推測,此情況可能使癌細胞對免疫療法產生抵抗力;不過,這項推論尚待進一步研究確認,若確定該情況對免疫療法有抵抗力,研究團隊也期望能深入研究如何逆轉這項抵抗力。 領導該研究的錫安山醫學中心醫學教授Ekihiro Seki表示,目前美國有25~30%成年人有肥胖問題, 其罹患脂肪肝的機率相當高;這項研究顯示,對於患有脂肪肝的大腸直腸癌患者,可能應採取不同 的治療方法。 而且,由於許多輕度脂肪肝可能未被檢測出,或尚未引起臨床醫師關注,Seki也敦促醫師應注意大 腸直腸癌病人中,可能罹患脂肪肝的患者。 他表示,在其團隊使用的患者樣本中,有超過40%患者患有脂肪肝,但醫師通常不會特別使用專門 檢測脂肪肝的磁振造影(MRI)進行檢查,意味著很多病例可能被遺漏。 此外,Seki也指出,對於亞洲族群中常見「體型偏瘦的脂肪肝患者」,仍須進一步研究,才能得知 其脂肪肝對腫瘤擴散的影響,是否與肥胖者相似。 參考資料: 1. 論文原文:https://www.cell.com/cell-metabolism/ppt/S1550-4131(23)00140-7.ppt#%20 禮來減重新藥臨床3期數據出爐!
雙重腸泌素促效劑72週體重降15.7% 美國時間27日,禮來(Eli Lilly)宣布,其雙重腸泌素促效劑tirzepatide對於938名肥胖和第2型糖尿病 患者的臨床3期試驗數據,受試者在治療72週後,體重減輕了15.6公斤(15.7%)。禮來將在未來幾周 內完成向美國食品藥物管理局(FDA)提交申請,以進行滾動式審查。 該臨床3期試驗名為SURMOUNT-2,是一項多中心、隨機、雙盲、安慰劑對照臨床試驗,比較10 mg和15 mg的tirzepatide與安慰劑,同時輔以低熱量飲食和增加運動,對於肥胖或超重、第2型糖 尿病患者的療效和安全性。 臨床試驗總共納入938名受試者,包含來自阿根廷、巴西、印度、日本、波多黎各、俄羅斯和台 灣等各國的患者,受試者在治療前的平均體重為100.7公斤,糖化血色素(Hb A1c)為8.0%, tirzepatide治療組的受試者以每4週增加2.5 mg間隔逐步增加劑量至最終維持10 mg或15 mg。 臨床結果顯示,安慰劑組體重平均減輕3.3%(約3.2公斤),而服用10 mg tirzepatide的受試者平均減 輕13.4%(約13.5公斤),服用15 mg tirzepatide的受試者平均減輕15.7%(約15.6公斤)。 體重至少減輕5%以上的受試者,在10 mg劑量組中高達81.6%,而在15 mg劑量組中佔86.4%,但 安慰劑組僅有30.5%。體重至少減輕15%以上的受試者,在10 mg劑量組中佔41.4%,而在15 mg劑 量組中佔51.8%,但安慰劑組僅有2.6%。 Tirzepatide治療組同時也達到所有關鍵次要臨床指標,包含降低Hb A1c與其他心臟代謝相關參數,與安慰劑組相比,tirzepatide治療組患者的HbA1c降低與針對第2型糖尿病患者的SURPASS臨 床試驗結果相似。 Tirzepatide治療的安全性與先前在SURMOUNT和SURPASS臨床試驗中相似,最常發生的不良事件 與腸胃道有關,嚴重程度為輕度至中度,通常發生在劑量遞增的期間,tirzepatide與安慰劑相比, 噁心、腹瀉、嘔吐和便秘的發生率較頻繁。 因不良事件而導致治療停止的佔比,在10 mg劑量組中佔3.8%,15 mg劑量組中佔7.4%,安慰劑組 中佔3.8%。 Tirzepatide先前在2022年5月獲批治療第二型糖尿病,該藥為雙重腸泌素(GLP-1和GIP)促效劑,是 十年來首個新類型的糖尿病藥物,也是首款可模擬GIP的藥物。 今年第一季,Mounjaro(tirzepatide)的銷售額達到5.68億美元,其中只有3200萬美元來自美國。分 析師在這次臨床數據公布後,認為該藥的銷售額可高達250億美元。 不過,禮來科學和醫學長Dan Skovronsky指出,tirzepatide就像其他藥物一樣,必須持續服用才能 有效果,目前,對於患者停藥後體重反彈的效應尚不清楚。 根據clinicaltrials.gov的資訊,禮來在本月21日,註冊了一項最新的臨床3b期試驗SURMOUNT-5, 預計在美國、加拿大等61個地點,招募700名肥胖或過重的受試者,進行Mounjaro(tirzepatide)與 諾和諾德(Novo Nordisk)的Wegovy (semaglutide)頭對頭(head-to-head)臨床試驗。該臨床試驗預 計將於2025年2月結束。 參考資料:https://investor.lilly.com/news-releases/news-release-details/lillys-tirzepatideachieved-157-weight-loss-adults-obesity-or https://www.fiercepharma.com/pharma/quitting-lillys-tirzepatide-may-be-difficult-manyobesity-patients (編譯/李林璦) https://news.gbimonthly.com/tw/article/show.php?num=58198&range=news 國家廣播公司(NBC)報導,食品暨藥物管理局(FDA)可望於今年內,批准禮來製藥(Eli Lilly)一款糖尿病藥物tirzepatide可以用於減肥,由於臨床實驗效果超過市面其他減肥藥,備受市場人士看好,預估可能成為有史以來最暢銷的減肥藥,但患者一年費用高達1.3萬元,目前沒有跡象顯示有保險公司會承保這款減肥藥。
這款名為「tirzepatide」的藥物一旦在今年內獲得FDA批准,將加入市場上另外兩種盛行、且價格高昂的減肥藥「Wegovy」和「Saxenda」行列,這款減肥藥均由諾和諾德藥廠(Novo Nordisk)生產。 上述三款減肥藥皆已證實對減肥有效,係透過注射一種叫做GLP-1的藥物,仿效減少食物攝取與降低食欲的激素,達到減肥效果。而禮來tirzepatide進一步模仿第二種稱為GIP的激素,降低食欲的同時、兼具改善身體分解醣分和脂肪的功效。 根據一項三期臨床實驗,高劑量的tirzepatide協助肥胖者平均減去約22.5%、亦即約52磅的體重,效果明顯高於市面其他減肥藥,在該項實驗中Wegovy和Saxenda分別減重約15%和5%。 市場人士預估禮來製藥可能採取和Wegovy和Saxenda類似的按月定價出售,Wegovy定價約每月1500美元而Saxenda定價則為約每月1350美元。臨床暨經濟評估研究所(the Institute for Clinical and Economic Review)醫療主管芮德(David Rind)推估,tirzepatide減肥藥其「公平價格」可能約每月1100美元或每年1.3萬元。 瑞士銀行(UBS)分析師布里斯托(Colin Bristow)樂觀預估tirzepatide未來年銷售額將達到250億元,可能超越艾伯維藥品(AbbVie)類風濕關節炎藥物「修美樂」(Humira)於2021年創下207億元的天文數字。 雖然禮來tirzepatide和Wegovy以及Saxenda此三款藥物皆已用於治療糖尿病,但是若用於減肥,保險公司可能不埋單,肥胖醫學專家史坦福(Fatima Stanford)博士表示,私人保險納入肥胖藥的承保範圍不一,尤其針對高價減肥藥更是如此,「唯一有能力負擔得起 tirzepatide減肥藥的人將是有錢人。」 from yahoo news 文╱藥師黃顗文
根據國民健康署研究,未婚女性的避孕方法中,口服避孕藥使用率從民國93年至105年由13.3%至20.1%,有上升的趨勢。 目前市面上的口服避孕藥為「雌激素Estrogen」和「黃體素Progestin」的複方成分,雌激素抑制排卵、減少濾泡發展、穩定子宮內膜和調節經血;黃體素抑制排卵、改變子宮頸黏液,和子宮內膜。雌激素可分為estrone(E1)、estradiol(E2)、estriol (E3)、estetrol (E4)。 其中Estetrol (E4) 是存在人體的天然雌激素,僅能在懷孕的女性中發現,且在生產之後會快速消失。E4於懷孕時,由E2、E3經由15α-hydroxylase與16α-hydroxylase於胎兒肝臟合成,再由胎盤循環至母體。E4生體可用率高達70%以上,半衰期約為28-32小時,是E2的兩倍長。其主要由肝臟經由phase II 代謝後,再由腎臟排除,並不會產生E1、E2、E3等活性代謝物,E4不會抑制或誘導CYP450,相較於E2,無顯著藥物交互作用。 與E2, E3相比,E4有較弱的雌激素活性,因其與estrogen receptor α (ERα)、β (ERβ)有較弱的親和力,且對於ERα有4-5倍的選擇性。不同於其他的雌激素,對於ERα均為促進(agonist)的作用,E4對在細胞核中的ERα是促進的作用,而細胞膜上的ERα則是拮抗效果。因上述作用機轉之特性,也觀測到在組織的不同效應:對應細胞核ERα為促進作用,E4在中樞神經系統有神經保護效果、抑制FSH、LH分泌,在骨頭能增加骨密度,減少骨質流失,在生殖系統能抑制排卵、子宮生長和上皮增殖;因對細胞膜ERα的拮抗作用,E4與E2相比,對於乳房的作用應較式微,目前研究並未觀測到會有乳房上皮細胞增長的作用。 E4/ DRSP(Drospirenone)的組合與EE / DRSP相比對於Activated Protein C等抗凝血因子等,較少有阻抗性效應。對於口服避孕藥最需要關注的安全性議題靜脈栓塞(VTE)的風險,或許E4/DRSP有較佳的安全性。進一步研究發現,推估E4/ DRSP的VTE發生率為3.66/10000 women-years;至於其他含DRSP避孕藥的發生率,根據一系統性回顧研究顯示,為7.8-9.3/10000 women-years。 E4不會與性激素結合球蛋白(sex hormone-binding globulin, SHBG)結合,E4/ DRSP與EE / DRSP相比對於SHBG影響較少,可能可以預期對於肝臟的影響較少;對於三酸甘油脂(TG)、低密度脂蛋白(LDL)等血脂數值的影響也較EE / DRSP少。與病人基礎值相比,E4/ DRSP無顯著影響膽固醇、高密度脂蛋白、低密度脂蛋白、三酸甘油脂。 此藥常見的副作用,如同其他現有的口服避孕藥,有情緒失調、非經期出血、陰道流血、乳房症狀、痤瘡、頭痛、體重增加、性慾降低等。 對於口服避孕藥使用,能多增加一項有人體來源的雌激素(E4),其獨特的選擇性作用機轉,不僅可有效避孕,且是預期有較佳安全性概況的新選擇。 (本文作者為馬偕紀念醫院藥師) 參考資料 1. Fruzzetti F, Fidecicchi T, Montt Guevara MM, Simoncini T. Estetrol: A New Choice for Contraception. J Clin Med. 2021;10(23):5625. 2. Lee A, Syed YY. Estetrol/Drospirenone: A Review in Oral Contraception. Drugs. 2022;82(10):1117-1125. https://www.taiwan-pharma.org.tw/weekly/2295/2295-4-1.htm 跨國特殊學名藥廠美時化學製藥與比利時女性健康用藥大廠Mithra Pharmaceuticals, SA(以下簡稱「Mithra」,布魯塞爾證券交易所股票代碼:MITRA)共同宣布,由Mithra研發之新一代口服避孕用藥Estelle已分別獲得台灣食品藥物管理署及香港衛生署藥物辦公室之核可,將以品牌名稱ALYSSA在亞洲市場行銷,美時擁有台灣與香港之獨家經銷權,已規劃2023年第一季於台灣市場推出,當年度在香港市場上市。
ALYSSA是全球第一個使用天然雌激素合成類似物雌四醇Estetrol(E4),與合併最新一代的黃體素Drospirenone(DRSP)之口服避孕用藥,並已在全球多個主要國家包含美國、加拿大、澳洲、與多個歐洲市場成功上市。 雌四醇E4是一種人體在懷孕時會自然產生的一種雌激素,而在ALYSSA中,此一雌四醇E4係由從植物中提煉出來的,再進行轉化合成。由於雌四醇E4獨特的藥理特性與作用機轉,被認為相對於現有的複合口服避孕藥,其治療風險應較低。ALYSSA不但具有絕佳的避孕效果,也兼具安全性及耐受性。 美時表示,在取得藥證後,將依照計畫時程依序在台灣及香港市場上市銷售。2021年度台灣口服避孕用藥市場規模約為美金1,400萬元,目前以人工合成之乙炔雌二醇(Ethinyl Estradiol)為基礎之複合口服用藥為大宗;而2021年度香港口服避孕用藥市場則約美金1,100萬元。 美時製藥總經理Petar Vazharov表示,我們很高興能與Mithra合作,在台灣和香港市場推出新一代口服避孕藥。繼我們現有的女性保健產品組合之後,ALYSSA在亞洲市場取證是為女性提供新一代健康選擇的重要里程碑。ALYSSA是一款有效、安全和耐受性良好的避孕藥,並且已證實對身體造成的影響較小。 Mithra製藥執行長Leon Van Rompay亦表示,我們很高興與合作夥伴美時一起分享Estelle 避孕藥在台灣和香港取得藥證的喜悅,這是Mithra在亞洲的首次授權。在美國、加拿大、澳洲和歐洲成功推出 Estelle之後,即將在這兩個市場以ALYSSA品牌推出這項產品,進一步證明了Mithra與核心市場優秀合作夥伴一起發展產品業務的能力。因此,我們很自豪能夠與美時攜手,持續進行避孕用藥領域的革新,為台灣和香港女性提供具有最佳效益/風險的替代選擇。 ►資料來源: http://www.investor.com.tw/onlineNews/NewsContent.asp?articleNo=14202209220029 財訊快報/何美如 相關減重藥品價格比較(以在美國的價格比較):
Wegovy(約1350美元{約台幣41850}) >Saxenda(約1300美元{約台幣40300}) >Rybelsus(約920美元{約台幣28520}) >Contrave(約550美元{約台幣17050}) >Qsymia(約200美元{約台幣6200}) 參考資料: Rybelsus in goodrx Saxenda in goodrx Contrave in goodrx Wegovy in goodrx Qsymia in goodrx 若有任何問題或需要領取相關藥物之疑問者,都歡迎加官方LINE帳號(點此即可加入)詢問。 2022最新美國腸胃科醫學會(ACG)減重藥物治療指引❗
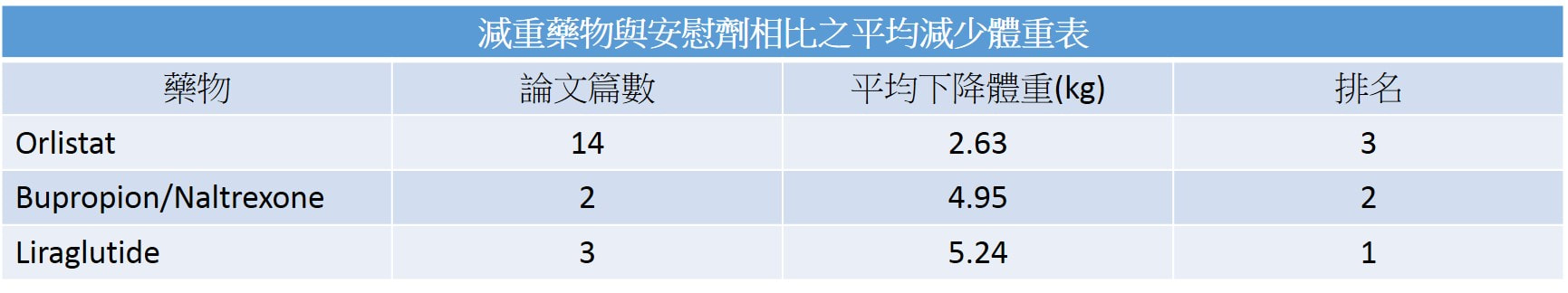
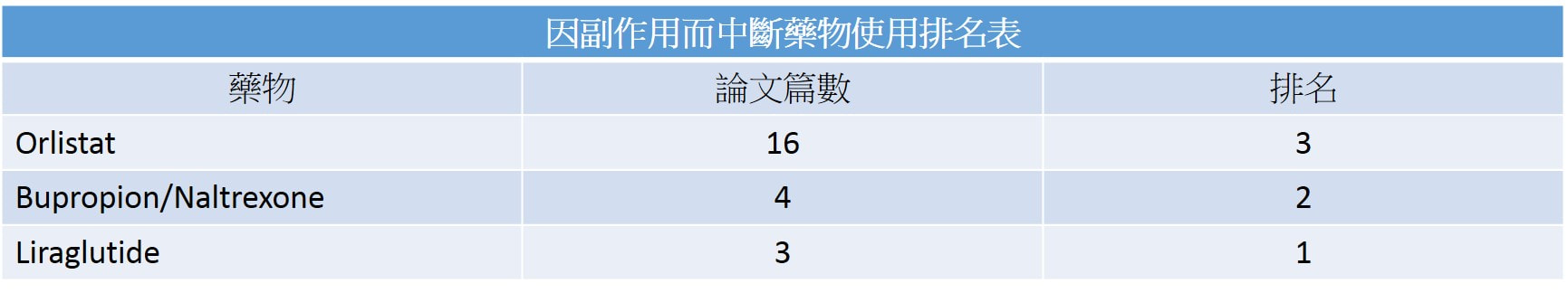
🦁美國腸胃科醫學會(ACG)在2022年10月底發表了最新的減重藥物治療指引,讓減重藥物的使用,在實證醫學上更確立了角色。文中提到,減重藥物搭配調整飲食生活習慣,對於肥胖且有共病的人,可以達到更好的減重效果。 文中提到使用的藥物有四種: 1️⃣ Semaglutide (Wegovy; 註: 目前台灣只有用於治療糖尿病的Ozempic, 胰妥讚及口服的Rybelsus瑞倍適) 2️⃣ Liraglutide (Saxenda, 善纖達) 3️⃣ Phentermine-topiramate (台灣未上市) 4️⃣ Naltrexone-bupropion (Contrave, 康纖芙) 根據文中指出,統合多篇臨床試驗資料,減重效果依序為semaglutide [10.8%] > phentermine-topiramate [8.5%] > liraglutide [4.8%] (善纖達) > Naltrexone-bupropion [3.0%] (康纖芙) [上述排列依臨床試驗平均下降體重%] 🧑⚕️減重藥物不是哪一個比較好,而是選擇適合自己的輔助減重藥品才是最重要的! [只是輔助,不是依賴] 例如:想要瘦比較多的可以選semaglutide;有調整每日食慾需求的可以選Saxenda;✌又想調控食慾又不喜歡打針或是也有戒菸需求的可以選Contrave 相關減重藥品價格比較:請查看下一篇文章 參考資料: Gastroenterology 2022;163:1198–1225 若有任何問題或需要領取相關藥物之疑問者,都歡迎加官方LINE帳號(點此即可加入)詢問。 噁心、便秘(19%)、頭痛(17%)、嘔吐、頭暈(10%)、失眠(9%)、口乾(8%)、腹瀉。
參考資料:康纖芙仿單 若有任何問題或需要領取相關藥物之疑問者,都歡迎加官方LINE帳號(點此即可加入)詢問。 台灣成人過重的盛行率,自2013年以來男女合計已超過40%;而國人十大死因中,與肥胖相關的疾病高達八項,肥胖成為國人不得不重視的健康議題。
當肥胖及三高患者至門診求助,應建議個案先改善生活習慣,也就是改變飲食及運動習慣,藉此減少熱量攝取、增加熱量消耗。然而減重後容易復胖,有研究證實這跟荷爾蒙和酵消化酵素的代償有關,使得減重相當不易。 臨床上若是病患BMI≧30kg/m2或是BMI≧27 kg/m2且至少有一種合併症(高血壓、第二型糖尿病或血脂異常),可使用減重藥物作為低卡飲食及適當運動的輔助治療。 目前三種TFDA認證的減重藥物
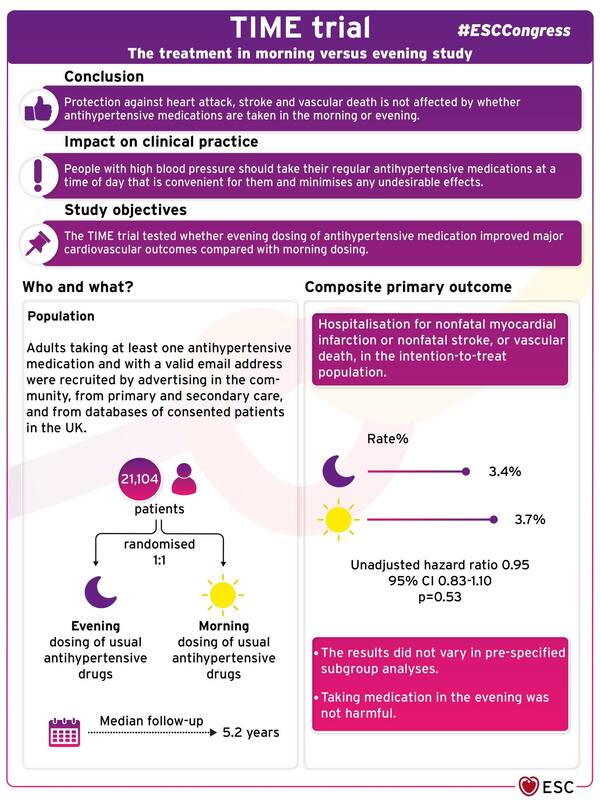
若有任何問題或需要領取相關藥物之疑問者,都歡迎加官方LINE帳號(點此即可加入)詢問。 參考資料: Obesity Management: Clinical Review and Update of the Pharmacologic Treatment Options Current treatments for obesity 雄性禿與圓禿有甚麼差別呢? 能治療圓禿,那能治療雄性禿嗎? Two Phase 3 Trials of Baricitinib for Alopecia Areata List of authors. Brett King, M.D., Ph.D., Manabu Ohyama, M.D., Ph.D., Ohsang Kwon, M.D., Ph.D., Abraham Zlotogorski, M.D., Justin Ko, M.D., Natasha A. Mesinkovska, M.D., Ph.D., Maria Hordinsky, M.D., Yves Dutronc, M.D., Wen-Shuo Wu, M.D., Jill McCollam, Pharm.D., Chiara Chiasserini, Sc.D., Guanglei Yu, Ph.D., et al., for the BRAVE-AA Investigators* Abstract BACKGROUND Alopecia areata is an autoimmune condition characterized by rapid hair loss in the scalp, eyebrows, and eyelashes, for which treatments are limited. Baricitinib, an oral, selective, reversible inhibitor of Janus kinases 1 and 2, may interrupt cytokine signaling implicated in the pathogenesis of alopecia areata. METHODS We conducted two randomized, placebo-controlled, phase 3 trials (BRAVE-AA1 and BRAVE-AA2) involving adults with severe alopecia areata with a Severity of Alopecia Tool (SALT) score of 50 or higher (range, 0 [no scalp hair loss] to 100 [complete scalp hair loss]). Patients were randomly assigned in a 3:2:2 ratio to receive once-daily baricitinib at a dose of 4 mg, baricitinib at a dose of 2 mg, or placebo. The primary outcome was a SALT score of 20 or less at week 36. RESULTS We enrolled 654 patients in the BRAVE-AA1 trial and 546 in the BRAVE-AA2 trial. The estimated percentage of patients with a SALT score of 20 or less at week 36 was 38.8% with 4-mg baricitinib, 22.8% with 2-mg baricitinib, and 6.2% with placebo in BRAVE-AA1 and 35.9%, 19.4%, and 3.3%, respectively, in BRAVE-AA2. In BRAVE-AA1, the difference between 4-mg baricitinib and placebo was 32.6 percentage points (95% confidence interval [CI], 25.6 to 39.5), and the difference between 2-mg baricitinib and placebo was 16.6 percentage points (95% CI, 9.5 to 23.8) (P<0.001 for each dose vs. placebo). In BRAVE-AA2, the corresponding values were 32.6 percentage points (95% CI, 25.6 to 39.6) and 16.1 percentage points (95% CI, 9.1 to 23.2) (P<0.001 for each dose vs. placebo). Secondary outcomes for baricitinib at a dose of 4 mg but not at a dose of 2 mg generally favored baricitinib over placebo. Acne, elevated levels of creatine kinase, and increased levels of low- and high-density lipoprotein cholesterol were more common with baricitinib than with placebo. CONCLUSIONS In two phase 3 trials involving patients with severe alopecia areata, oral baricitinib was superior to placebo with respect to hair regrowth at 36 weeks. Longer trials are required to assess the efficacy and safety of baricitinib for alopecia areata. (Funded by Eli Lilly under license from Incyte; BRAVE-AA1 and BRAVE-AA2 ClinicalTrials.gov numbers, NCT03570749. opens in new tab and NCT03899259. opens in new tab.) 參考資料: Two Phase 3 Trials of Baricitinib for Alopecia Areata 降血壓藥到底白天吃要比較好還是晚上吃比較好?Evening dosing of blood pressure medication not better than morning dosing8/29/2022 降血壓藥到底白天吃要比較好還是晚上吃比較好?
這個問題一直以來都個有說法,一直到最近的研究總算有個比較清楚的結論。 那就是一樣好。 降血壓藥在白天吃跟在晚上吃一樣。 TIME trial presented in a Hot Line Session today at ESC Congress 2022 26 Aug 2022 Topic(s): Cardiovascular PharmacotherapyBarcelona, Spain – 26 Aug 2022: A pragmatic randomised trial in more than 21,000 patients with high blood pressure followed for over five years has concluded that protection against heart attack, stroke and vascular death is not affected by whether antihypertensive medications are taken in the morning or evening. The late breaking research is presented in a Hot Line session today at ESC Congress 20221 and contradicts previous findings that suggested a very large cardiovascular benefit of night-time dosing.2 More than one billion people have high blood pressure worldwide.3 High blood pressure is the leading global cause of premature death, accounting for almost ten million deaths in 2015, of which 4.9 million were due to ischaemic heart disease and 3.5 million were due to stroke. Nocturnal blood pressure is a better predictor of cardiovascular outcomes than daytime blood pressure, and there is previous evidence that antihypertensive drugs taken in the evening rather than in the morning reduced night-time blood pressure to a greater extent.4 The Hygia study2 previously indicated a protective effect of nocturnal dosing on cardiovascular events, but this study has attracted criticism.5 TIME was a large prospective, randomised trial conducted to test whether evening dosing of antihypertensive medication improved major cardiovascular outcomes compared with morning dosing.6 Adults taking at least one antihypertensive medication and with a valid email address were recruited by advertising in the community, from primary and secondary care, and from databases of consented patients in the UK. After participants had signed up on the TIME website (http://www.timestudy.co.uk) and their eligibility was confirmed, they were randomised 1:1 to take their usual antihypertensive medication in the morning or the evening. The composite primary endpoint was hospitalisation for nonfatal myocardial infarction or nonfatal stroke, or vascular death, in the intention-to-treat population. Information on hospitalisations and deaths was obtained from participants by email and through record linkage to national databases and further data was gathered from family doctors and hospitals and independently adjudicated by a committee blinded to allocated dosing time. A total of 21,104 patients were randomised, 10,503 to evening dosing and 10,601 to morning dosing. The average age of participants was 65 years, 58% were men and 98% were white. The median follow-up duration was 5.2 years but some were in the study for over nine years. The primary endpoint occurred in 362 (3.4%) participants in the evening dosing group (0.69 events per 100 patient years) and 390 (3.7%) in the morning dosing group (0.72 events per 100 patient years), giving an unadjusted hazard ratio of 0.95 (95% confidence interval 0.83–1.10; p=0.53). The results did not vary in pre-specified subgroup analyses. Taking medication in the evening was not harmful. Principal investigator Professor Thomas MacDonald of the University of Dundee, UK said: “TIME was one of the largest cardiovascular studies ever conducted and provides a definitive answer on the question of whether blood pressure lowering medications should be taken in the morning or evening. The trial clearly found that heart attack, stroke and vascular death occurred to a similar degree regardless of the time of administration. People with high blood pressure should take their regular antihypertensive medications at a time of day that is convenient for them and minimises any undesirable effects.” ENDS 參考資料: Evening dosing of blood pressure medication not better than morning dosing 一次簡單搞懂減重藥「康纖芙」 CONTRAVE(BUPROPION AND NALTREXONE)
ContraveⓇ(bupropion/naltrexone)是2014年FDA核准上市的複方製劑,適合同時需要戒菸與減重者,不建議當作減重第一線藥物。Bupropion是非典型抗憂鬱劑,naltrexone是單純鴉片類受體拮抗劑,bupropion/naltrexone確實的作用機轉尚未被完全了解,可能是藉由增加下視丘促黑素皮質素神經元(食慾調節中心)的放電速率來調節減少食物的攝取。 Bupropion/naltrexone減重效果與lorcaserin相似,但是有較多的副作用。研究指出,相較於安慰劑組,服用bupropion/naltrexone可以顯著降低體重(-5~6% vs -1.3%),但是血壓上升與心跳快的副作用比例也較高。常見副作用有噁心(30%)、頭痛(14%)及便秘(15%)等。患有癲癇、未良好控制的高血壓、長期使用鴉片藥物、14天內使用過MAOI藥品者應避免使用。 參考資料: FDA 藥師週刊 Naltrexone/Bupropion ER (Contrave) 此文章詳細內容依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。 恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 若有任何問題或需要領取相關藥物之疑問者,都歡迎加官方LINE帳號(點此即可加入)詢問。 一次簡單搞懂圓形禿新藥OLUMIANT (BARICITINIB)
此藥品原為治療類風濕性關節炎。 參考資料: FDA FDA Approves First Systemic Treatment for Alopecia Areata 此文章詳細內容依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。 恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 友華進軍減重市場 新機轉口服新藥獲TFDA核准 (圖為友華生技執行長蔡孟霖,圖片來源:本刊資料中心)
日前,友華生技(4120)引進美國最新機轉減重口服藥Contrave「康纖芙」,三月份剛取得國內衛福部藥證,預計今年下半年上市,這是友華進軍減重市場的首支減重產品。 友華去年與康霈生技合作代理CBL-514溶脂注射劑新藥,今年取得口服新藥的藥證,友華生技執行長蔡孟霖表示,Contrave「康纖芙」的作用機轉在於能將降低飢餓感與對食物的渴望,達到減重目標,根據國外文獻報告,在第四週起便可達到較安慰劑更顯著的體重減輕,是國內最新機轉的口服新藥,上市後將提供全新治療選擇。 Contrave「康纖芙」於2014年就陸續在美國、歐盟、加拿大及澳洲核准上市,也已在亞洲新加坡及韓國上市,今年國內上市核准用於體重控制,做為低熱量飲食、以及增加體能活動的輔助療法,適用於身體質量指數BMI≧30的肥胖患者,或是BMI≧27、且病人至少有一項體重相關共病症。 上述文字引述環球生技 6 月 13 日,美國食品藥物管理局(FDA)宣佈核准禮來(Eli Lilly)的 Olumiant (baricitinib) 口服藥物,用於治療患有嚴重圓禿(alopecia areata)成年患者。圓禿是一種常見因自體免疫異常導致的皮膚落髮症狀。 FDA 核准 Olumiant 的關鍵進展,帶來首個圓禿的全身性治療(systemic treatment)而非局部性治療方法。FDA 皮膚科藥物評估和研究中心 Kendall Marcus 主任表示,此項批准有助於滿足嚴重圓禿者的重大未滿足治療需求。 JAK 抑制劑可減緩毛囊發炎、減少落髮 圓禿是一種自體免疫疾病,因為免疫系統的淋巴球異常攻擊毛囊,毛囊急性發炎導致的落髮現象。全美每年約有超過 30 萬人飽受圓禿所擾,知名影帝 Will Smith 妻子 Jada Pinkett Smith 也是患者之一。 現有圓禿療法包含局部塗抹類固醇藥物,產生抗發炎作用,例如嬌生(J&J)的 Rogaine,其含有 minoxidil 也是生髮水中主要成分;另外有些類固醇藥物則是注射劑形式。而 Olumiant 是一種 JAK 抑制劑,透過阻斷發炎反應的酵素活性達到減緩落髮效果。 兩項 Olumiant 的臨床試驗(Trial AA-1, Trial AA-2),招募落髮受試者為落髮至少 50% 達 6 個月以上患者,他們每天分別接受安慰劑、2/4 mg Olumiant 治療。Trial AA-1 結果顯示 2/4 mg Olumiant 治療組分別有 22%、35% 獲得足夠的頭髮覆蓋,安慰劑組僅 5%。Trial AA-2 中 2/4 mg Olumiant 治療組分別有 17%、32% 獲得足夠的頭髮覆蓋,安慰劑組僅 3%。整體而言,至少一半以上受試者在治療第 36 週時,出現 80% 以上頭髮覆蓋改善情形。 Olumiant 相繼核准用於類風濕關節炎、COVID-19 治療 Olumiant 是禮來與美國公司 Incyte 自 2008 年起授權合作,共同研發的 JAK 抑制劑藥物,目前已成功累積數項適應症。 2018 年 6 月,FDA 首度批准 Olumiant 作為成年中至重度類風濕關節炎(rheumatoid arthritis)療法。適用對象是以接受過一種以上 TNF 抑制劑者,需特別注意的是 Olumiant 不可與其他 JAK 抑制劑聯用。 今年 5 月,FDA 再許可 Olumiant 用於住院且需氧氣支持的特定重度新冠患者。先前 FDA 已通過Olumiant 的緊急使用授權(EUA),正式核准後 Olumiant 則成為第一個、且是唯一一款重症新冠的 JAK 抑制劑藥物。 同時,近年 FDA 對 JAK 抑制劑藥物也給予相當關注,揭露該類免疫抑制藥物可能有引發心臟病、中風等副作用風險。去年 FDA 公布更新 3 款 JAK 抑制劑藥物的黑盒警示(black box warning),除了包含禮來的 Olumiant,另外還有輝瑞(Pfizer)的 Xeljanz、與艾伯維(Abbvie)的 Rinvoq;Xeljanz、Rinvoq 都是類風濕關節炎用藥。 上述文字來自GENE ONLINE 以下內容僅轉載Healio.com 不代表新德大藥局之任何意見以下內容摘自Healio.com SURMOUNT-1: Adults achieve weight loss of 16% or more at 72 weeks with tirzepatide Adults with overweight or obesity taking the once-weekly GIP/GLP-1 receptor agonist tirzepatide achieved a mean weight loss of at least 16% at 72 weeks, according to topline results from the SURMOUNT-1 clinical trial. In a phase 3 trial with 2,539 participants, tirzepatide (Eli Lilly) met both primary endpoints for mean percentage change in body weight from baseline and percentage of participants with a 5% reduction in body weight compared with placebo. Adults randomly assigned to receive tirzepatide had a mean weight loss of 16% with 5 mg, 21.4% with 10 mg, and 22.5% with 15 mg compared with a 2.4% mean weight loss for those assigned placebo. Of those assigned tirzepatide, 89% achieved a weight loss of at least 5% compared with 28% in the placebo group. Louis J. Aronne, MD, FACP, DABOM Aronne is the Sanford I. Weill Professor of Metabolic Research at Weill Cornell Medicine at New York-Presbyterian/Weill Cornell Medical Center and investigator in the SURMOUNT-1 trial. “This study shows that highly significant weight loss, in line with what is achieved with surgical procedures, can be achieved with tirzepatide,” Louis J. Aronne, MD, FACP, DABOM, the Sanford I. Weill Professor of Metabolic Research at Weill Cornell Medicine at New York-Presbyterian/Weill Cornell Medical Center and investigator in the SURMOUNT-1 trial, told Healio. “The mean BMI was about 38 kg/m2 to start and was reduced to about 30 kg/m2 with many subjects getting into the normal range.” Tirzepatide met all key secondary endpoints, with 55% of those receiving 10 mg and 63% of those receiving 15 mg achieving a body weight reduction of at least 20% compared with 1.3% of the placebo group. In an additional secondary endpoint analysis not controlled for type 1 error, 32% of those receiving 5 mg tirzepatide lost at least 20% of body weight, according to the release. “For perspective, a 5% weight loss reduces the risk of developing diabetes by 50%, and a 10% weight loss reduces it by 80%,” Aronne said. “Lap band produces about 17% weight loss, and sleeve gastrectomy 25% mean weight loss. The gap between diets and bariatric surgery is being filled by medical therapy.” The safety and tolerability profile for tirzepatide was similar to that those of other incretin-based therapies approved for obesity treatment. Most side effects were mild to moderate; the most common were nausea, diarrhea, constipation and vomiting. Participants with prediabetes at the start of the trial will remain enrolled for an additional 104 weeks beyond the initial 72-week trial period to evaluate the impact of body weight and potential differences in type 2 diabetes progression with tirzepatide compared with placebo. The SURMOUNT-1 results will be presented at an upcoming medical meeting and submitted to a peer-reviewed journal, according to the release. Additional studies are ongoing. 根據 SURMOUNT-1 臨床試驗的一線結果,每週服用一次 GIP/GLP-1 受體激動劑替西帕肽的超重或肥胖成人在 72 週時平均體重減輕至少 16%。
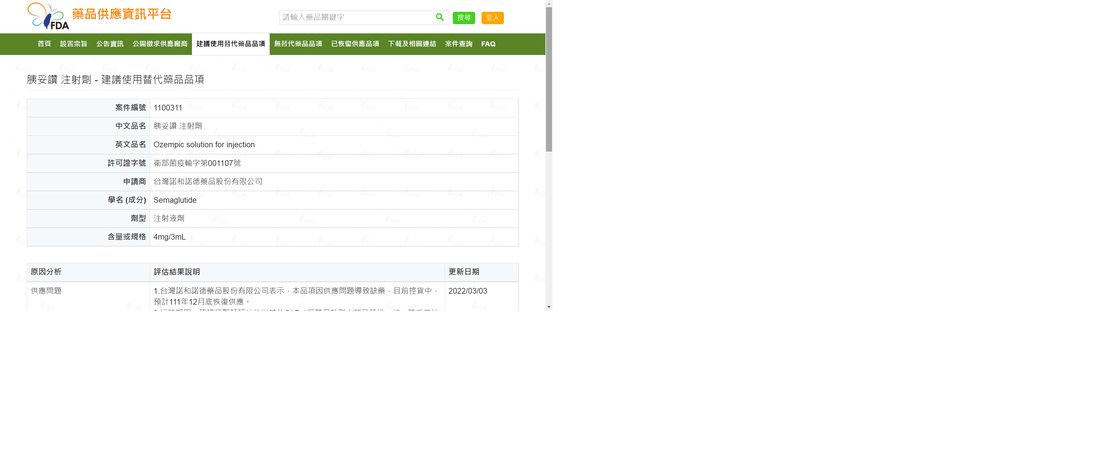
在一項有 2,539 名參與者的 3 期試驗中,tirzepatide (Eli Lilly) 達到了兩個主要終點,即體重相對於基線的平均百分比變化和與安慰劑相比體重減輕 5% 的參與者百分比。隨機分配接受 tirzepatide 的成年人平均體重減輕 16%(5 毫克)、21.4%(10 毫克)和 22.5%(15 毫克),而安慰劑組平均體重減輕 2.4%。在分配替西帕肽的患者中,89% 的患者體重減輕至少 5%,而安慰劑組為 28%。 “這項研究表明,使用 tirzepatide 可以顯著減輕體重,與外科手術所達到的效果一致,”威爾康奈爾大學 Sanford I. Weill 代謝研究教授Louis J. Aronne 醫學博士、FACP、DABOM紐約長老會/威爾康奈爾醫學中心的醫學和 SURMOUNT-1 試驗的調查員告訴 Healio。“開始時的平均 BMI 約為 38 kg/m 2 ,隨著許多受試者進入正常範圍,隨後降至約 30 kg/m 2。 ” Tirzepatide 達到了所有關鍵的次要終點,55% 的接受 10 mg 的患者和 63% 的接受 15 mg 的患者實現了至少 20% 的體重減輕,而安慰劑組為 1.3%。根據發布的消息,在另一項未對 1 型錯誤進行控制的次要終點分析中,接受 5 mg 替西帕肽的患者中有 32% 的人體重減輕了至少 20%。 “從長遠來看,減重 5% 可將患糖尿病的風險降低 50%,減重 10% 可降低 80%,”Aronne 說。“膝帶可減輕約 17% 的體重,袖狀胃切除術平均減輕 25% 的體重。飲食和減肥手術之間的差距正在通過藥物治療來填補。” 替西帕肽的安全性和耐受性與其他批准用於肥胖治療的腸促胰島素療法相似。大多數副作用為輕度至中度;最常見的是噁心、腹瀉、便秘和嘔吐。 在試驗開始時患有前驅糖尿病的參與者將在最初的 72 週試驗期之後再加入 104 週,以評估體重的影響以及 tirzepatide 與安慰劑相比對 2 型糖尿病進展的潛在差異。 據新聞稿稱,SURMOUNT-1的結果將在即將召開的醫學會議上公佈,並提交給同行評審的期刊。其他研究正在進行中。 此文章詳細內容依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。 恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 OZEMPIC 4MG/3ML SOLUTION FOR INJECTION胰妥讚注射劑藥品缺藥 台灣諾和諾德藥品股份有限公司表示,本品項因供應問題導致缺藥,預計111年12月底恢復供應。 因此,請慢性處方箋上有此品項者,到原處方醫院領取慢性處方箋。 造成困擾,敬請見諒。 另外,目前有很多人使用胰妥讚作為減重用藥,甚至在沒有醫師開立處方下,就私下使用,目前胰妥讚的藥證內容只限於糖尿病患者用藥,並沒有經衛福部核可做為
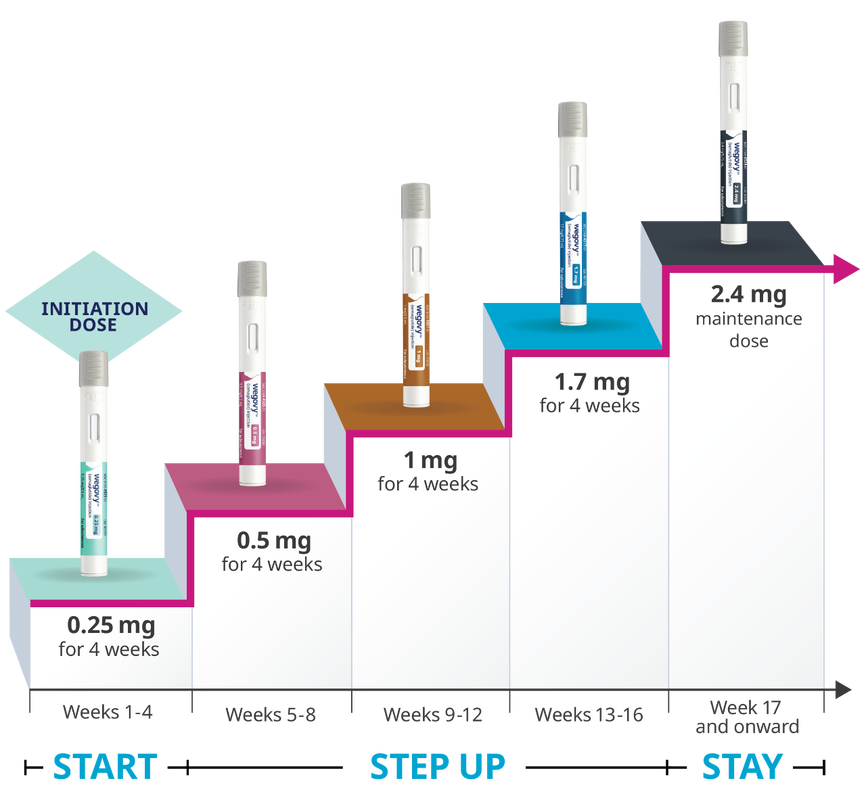
"用於體重控制,做為低熱量飲食及增加體能活動外之輔助療法。" 因此,即使是醫師開立胰妥讚做為減重輔助藥品,也算是藥品仿單標示外之適應症使用。 至於甚麼是藥品仿單標示外之適應症使用,可以參考以下影片或文章。 影片:一次搞懂藥品仿單標示外使用(Off Label Use) 文章:一次搞懂藥品仿單標示外使用(Off Label Use) 若有任何問題或需要領取相關藥物之慢性處方箋者,都歡迎加官方LINE帳號(點此即可加入)詢問。 FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014 (自 2014 年以來,FDA 首次批准用於慢性體重管理的新藥治療) Wegovy 於2021 年 6 月 4 日通過FDA許可作為減重的針劑。 詳細內容可以參考FDA的文章或是諾和諾德的官方網站。 此文章詳細內容依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。
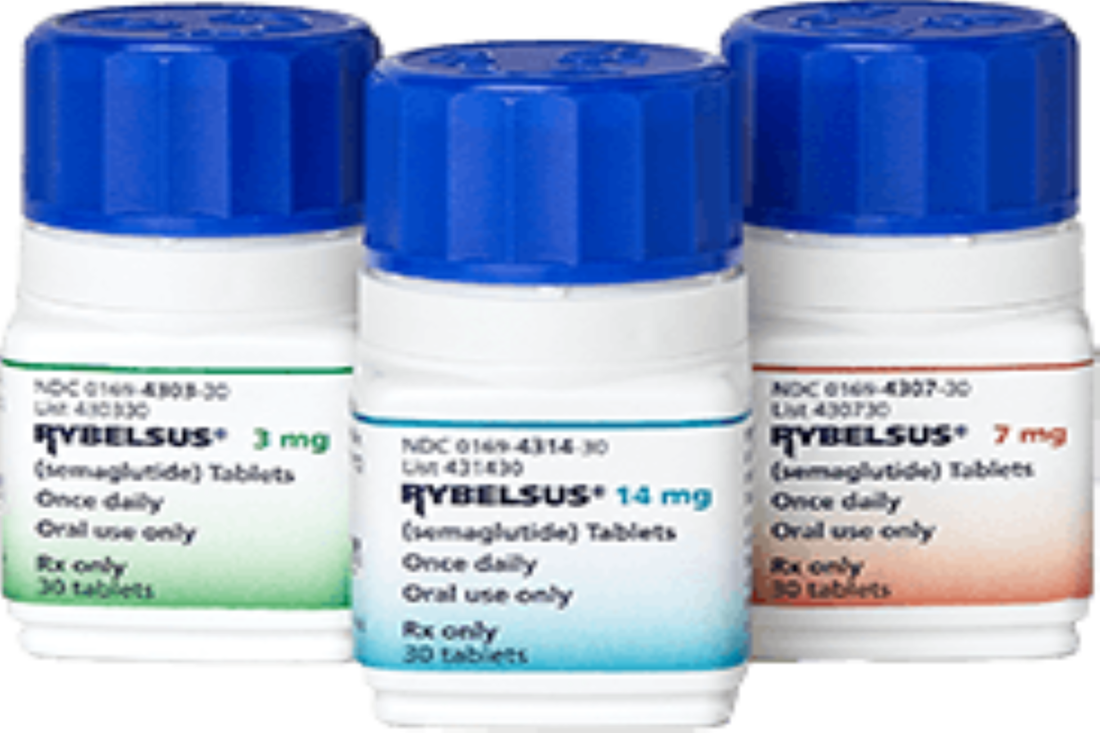
恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 Rybelsus 瑞倍適(semaglutide)在2019年9月20日通過美國FDA的藥品許可,成為全世界第一個口服的GLP-1降血糖藥。
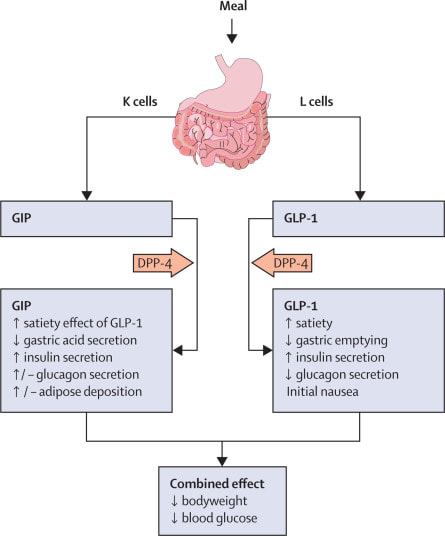
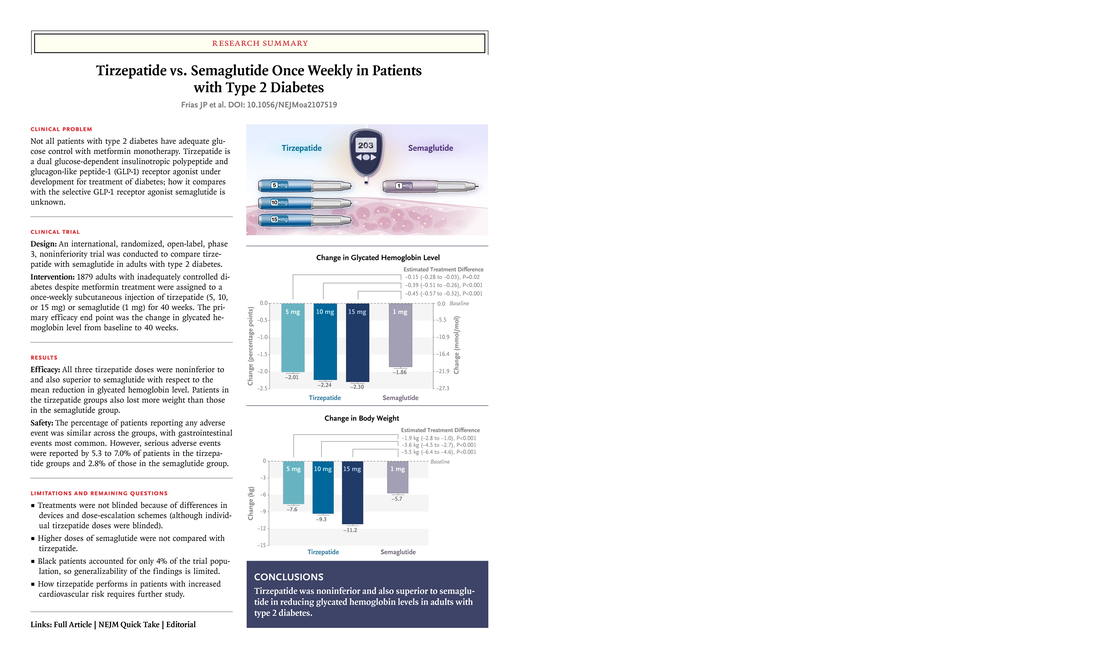
目前核可的適應症為第二型糖尿病的用藥,未來在減重輔助治療的臨床試驗中,有機會得到不錯的臨床數據。 同成分的針劑: 1、OZEMPIC於2017年12月5日取得第二型糖尿病的適應症。 2、WEGOVY於2021年6月4日取得減重輔助治療適應症。 參考資料: FDA FDA approves first oral GLP-1 treatment for type 2 diabetes FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014 此文章詳細內容依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。 恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 結論 同樣一週一次注射的Tirazepatide與Semaglutide比較,Tirazepatide能下降更多的血糖與體重,而副作用並沒有高更多。 附註:禮來(Eli Lilly) 預計2022年4月對於tirzepatide之關鍵臨床試驗發布結果。 參考資料: 1、Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes 2、‘Stunning’ Twincretin Beats Semaglutide in Type 2 Diabetes 此文章詳細內容依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。 恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 近期有太多人詢問
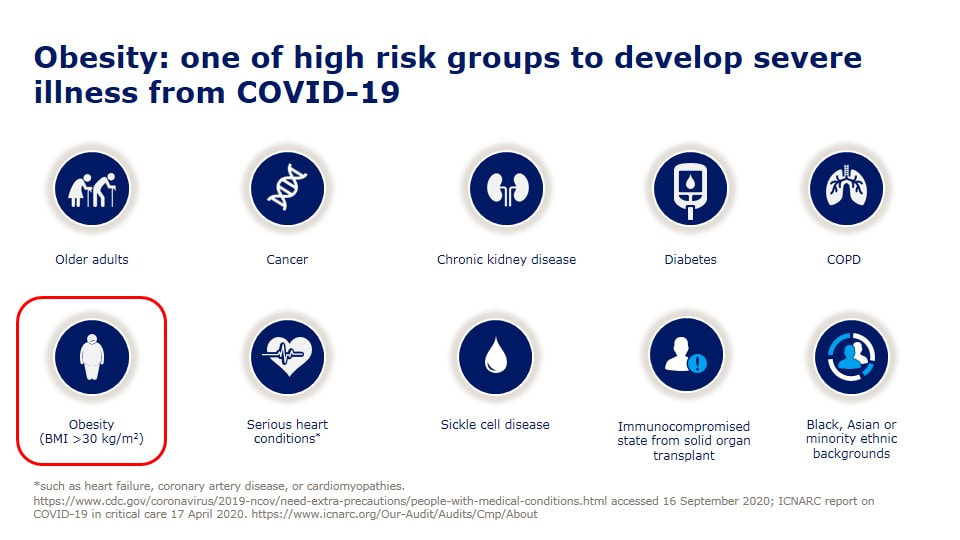
以SEMAGLUTIDE成份 核准的藥品包含 1、針劑:OZEMPIC 宜妥讚 2、口服藥:RYBELSUS 瑞倍適錠 到底可否用來減肥(重)呢? 當然更多人詢問, 到底在臨床上用來減重的效果好不好? 此文章依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。 恕不對外開放非專業人士使用。 若有任何問題或需要驗證通過專業人員區,都歡迎加官方LINE帳號(點此即可加入)詢問。 今天看到死亡個案出現一個「無慢性病史」的30幾歲女性,怎麼會這樣呢? 沒多久就就看到這個報導, 30多歲女「快速死」PCR陰轉陽5天不治 體重90公斤致病情惡化 「肥胖症」其實早就被世界衛生組織正名是一種「脂肪相關慢性疾病」(Adiposity-Based Chronic Disease (ABCD) )積極治療肥胖根本不是外觀的問題,而是跟治療高血壓、糖尿病一樣,刻不容緩的事情。 在今年四月前新冠死亡率居全球之冠的捷克,每10萬人就有229人染疫喪命,而捷克的肥胖是全歐洲之冠,女性57%肥胖、男性71%肥胖,10個新冠患者有8個肥胖, 新冠死亡率被認為跟肥胖有明確且嚴重的相關性。 台灣目前是亞洲的肥胖冠軍........ 一些區域的研究,發現糖尿病會讓新冠病毒死亡的風險多了8倍,而肥胖可以讓死亡風險高達10倍。 如果控制血糖跟血壓很重要,那控制體重難道不是更重要嗎? 研究證實,當肥胖者減少5%以上體重(如成人90公斤,減少5公斤),就可以為健康帶來許多益處,高血壓、糖尿病等與肥胖相關疾病將可改善。 強烈建議以後新冠肺炎死亡病例的「慢性病史」,麻煩一定要把「肥胖」列入在裡面,才能讓民眾正視自己的健康問題! 對於醫療人員來說處理肥胖,也是非常緊急的事,當BMI超過25時,每增加1單位,相對所有原因死亡率風險便增加9%,包括心因性猝死風險,是的,先不提快樂缺氧了,每個BMI在30以上的人,我都會嚴正告訴他猝死的風險,千萬不要因為自己年輕、或是血液報告尚可,就忽視了隱藏的風險。 也可以參考美國疾病管制局 www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html OverviewAdults of any age with the following conditions can be more likely to get severely ill from COVID-19. Severe illness means that a person with COVID-19 may need:
Preventive measures for COVID-19 (including vaccination, wearing a mask and social distancing) are important especially if you are older or have multiple or severe health conditions. You can learn about CDC’s COVID-19 vaccine recommendations, including how medical conditions and other factors inform recommendations, here. Note: The list below does not include all potential medical conditions that could make you more likely to get severely ill. Rare medical conditions may not be included below. However, a person with a condition that is not listed may still be in more danger from COVID-19 than persons of similar age who do not have the condition and should talk with their healthcare provider. Top of PageMedical Conditions in Adults
Get more information:
Get more information: Chronic lung diseases, including COPD (chronic obstructive pulmonary disease), asthma (moderate-to-severe), interstitial lung disease, cystic fibrosis, and pulmonary hypertensionChronic lung diseases can make you more likely to get severely ill from COVID-19. These diseases may include:
Get more information: Diabetes (type 1 or type 2)Having either type 1 or type 2 diabetes can make you more likely to get severely ill from COVID-19. Get more information:
Get more information: Heart conditions (such as heart failure, coronary artery disease, cardiomyopathies or hypertension)Having heart conditions such as heart failure, coronary artery disease, cardiomyopathies, and possibly high blood pressure (hypertension) can make you more likely to get severely ill from COVID-19. Get more information: HIV infectionHaving HIV (Human Immunodeficiency Virus) can make you more likely to get severely ill from COVID-19. Get more information: Immunocompromised state (weakened immune system)Having a weakened immune system can make you more likely to get severely ill from COVID-19. Many conditions and treatments can cause a person to be immunocompromised or have a weakened immune system. Primary immunodeficiency is caused by genetic defects that can be inherited. Prolonged use of corticosteroids or other immune weakening medicines can lead to secondary or acquired immunodeficiency. Get more information:
Get more information:
Get more information:
Get more information:
Get more information: Smoking, current or formerBeing a current or former cigarette smoker can make you more likely to get severely ill from COVID-19. If you currently smoke, quit. If you used to smoke, don’t start again. If you’ve never smoked, don’t start. Get more information:
Get more information: Stroke or cerebrovascular disease, which affects blood flow to the brainHaving cerebrovascular disease, such as having a stroke, can make you more likely to get severely ill from COVID-19. Get more information: Substance use disordersHaving a substance use disorder (such as alcohol, opioid, or cocaine use disorder) can make you more likely to get severely ill from COVID-19. Get more information:
Actions You Can TakeIn general, the older you are, the more health conditions you have, and the more severe the conditions, the more important it is to take preventive measures for COVID-19 such as vaccination, wearing a mask , social distancing, and practicing hand hygiene. Please contact your state, tribal, local, or territorial health department for more information on COVID-19 vaccination in your area. It is important for people with medical conditions and their providers to work together and manage those conditions carefully and safely. Get a COVID-19 vaccine as soon as you can. If you have a medical condition, the following are actions you can take based on your medical conditions and other risk factors:
美國食品及藥物管理局(FDA)7日通過首款有助於減緩阿茲海默症(Alzheimer's disease)認知退化的藥物。研究證明,美國藥廠百健(Biogen)研發、名稱為Aducanumab的新藥,可以讓阿茲海默症引起的輕度認知障礙及早期失智症(dementia),雙雙獲得改善。根據估計,每名阿茲海默症患者使用Aducanumab,一年下來估計花費為5萬元。 華盛頓郵報指出,這是第一款證實可以減緩腦部功能退化而獲得食藥局通過的新藥,不只是僅僅能夠舒緩症狀而已。從2003年以來,並沒有任何阿茲海默症獲得食藥局通過,由此可見阿茲海默症藥物研發失敗率極高。 Aducanumab新藥研發過程中,曾引發正反兩派論戰。支持者認為,這款藥品獲得食藥局核可之後,可望引來各界對於阿茲海默症更高關注,進而帶動對於治療阿茲海默症解方的更多研究與投資。 百健藥廠表示,Aducanumab的問世能讓阿茲海默症患者爭取到更多寶貴時間與家人相處,患者的日常生活也可以自理,例如清潔、購物等。 Aducanumab是實驗室研發的蛋白質「單株抗體」(monoclonal antibody),必須透過靜脈注射,啟動患者體內的反疫反應,清除腦部澱粉蛋白斑塊。百健藥廠強調,這款藥品並不會治好阿茲海默症。 然而,反對派人士則表示,Aducanumab藥品是否確實有效,數據資料薄弱,食藥局為這款新藥放行,反映出主管機關迫於病患及倡議團體壓力之下,降低審查標準。 「阿茲海默症協會」(Alzheimer's Association)指出,美國目前約有620萬名阿茲海默症患者,如果沒有突破療法出現,患者人數到了2050年可望翻倍。 文章來源:www.worldjournal.com/wj/story/121469/5516439 FDA’s Decision to Approve New Treatment for Alzheimer’s Disease By Dr. Patrizia Cavazzoni, Director, FDA Center for Drug Evaluation and Research
Today FDA approved Aduhelm (aducanumab) to treat patients with Alzheimer’s disease using the Accelerated Approval pathway, under which the FDA approves a drug for a serious or life-threatening illness that may provide meaningful therapeutic benefit over existing treatments when the drug is shown to have an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients and there remains some uncertainty about the drug’s clinical benefit. This approval is significant in many ways. Aduhelm is the first novel therapy approved for Alzheimer’s disease since 2003. Perhaps more significantly, Aduhelm is the first treatment directed at the underlying pathophysiology of Alzheimer’s disease, the presence of amyloid beta plaques in the brain. The clinical trials for Aduhelm were the first to show that a reduction in these plaques—a hallmark finding in the brain of patients with Alzheimer’s—is expected to lead to a reduction in the clinical decline of this devastating form of dementia. We are well-aware of the attention surrounding this approval. We understand that Aduhelm has garnered the attention of the press, the Alzheimer’s patient community, our elected officials, and other interested stakeholders. With a treatment for a serious, life-threatening disease in the balance, it makes sense that so many people were following the outcome of this review. Further, the data included in the applicant’s submission were highly complex and left residual uncertainties regarding clinical benefit. There has been considerable public debate on whether Aduhelm should be approved. As is often the case when it comes to interpreting scientific data, the expert community has offered differing perspectives. At the end of the day, we followed our usual course of action when making regulatory decisions in situations where the data are not straightforward. We examined the clinical trial findings with a fine-tooth comb, we solicited input from the Peripheral and Central Nervous System Drugs Advisory Committee, we listened to the perspectives of the patient community, and we reviewed all relevant data. We ultimately decided to use the Accelerated Approval pathway—a pathway intended to provide earlier access to potentially valuable therapies for patients with serious diseases where there is an unmet need, and where there is an expectation of clinical benefit despite some residual uncertainty regarding that benefit. In determining that the application met the requirements for Accelerated Approval, the Agency concluded that the benefits of Aduhelm for patients with Alzheimer’s disease outweighed the risks of the therapy. What the Data Show The late-stage development program for Aduhelm consisted of two phase 3 clinical trials. One study met the primary endpoint, showing reduction in clinical decline. The second trial did not meet the primary endpoint. In all studies in which it was evaluated, however, Aduhelm consistently and very convincingly reduced the level of amyloid plaques in the brain in a dose- and time-dependent fashion. It is expected that the reduction in amyloid plaque will result in a reduction in clinical decline. We know that the Peripheral and Central Nervous System Drugs Advisory Committee, which convened in November 2020 to review the clinical trial data and discuss the evidence supporting the Aduhelm application, did not agree that it was reasonable to consider the clinical benefit of the one successful trial as the primary evidence supporting approval. The option of Accelerated Approval was not discussed by the Advisory Committee. As mentioned above, treatment with Aduhelm was clearly shown in all trials to substantially reduce amyloid beta plaques. This reduction in plaques is reasonably likely to result in clinical benefit. After the Advisory Committee provided its feedback, our review and deliberations continued, and we decided that the evidence presented in the Aduhelm application met the standard for Accelerated Approval. We thank the Advisory Committee for its independent review of the data and valuable advice. Accelerated Approval The FDA instituted its Accelerated Approval Program to allow for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need. Approval is based on a surrogate or intermediate clinical endpoint (in this case reduction of amyloid plaque in the brain). A surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. The use of a surrogate endpoint can considerably shorten the time required prior to receiving FDA approval. Drug companies are required to conduct post-approval studies to verify the anticipated clinical benefit. These studies are known as phase 4 confirmatory trials. If the confirmatory trial does not verify the drug’s anticipated clinical benefit, FDA has regulatory procedures in place that could lead to removing the drug from the market. The Devastation of Alzheimer’s Disease With all this said, we are extremely aware of the gradual and cumulative devastation that Alzheimer’s disease causes, as patients lose their memory and cognitive functioning over time. In late-stage disease, people can no longer hold a conversation or respond to their environment. On average, a person with Alzheimer’s disease lives four to eight years after diagnosis, but some patients can live up to 20 years with the disease. The need for treatments is urgent: right now, more than 6 million Americans are living with Alzheimer’s disease and this number is expected to grow as the population ages. Alzheimer's is the sixth leading cause of death in the United States. Although the Aduhelm data are complicated with respect to its clinical benefits, FDA has determined that there is substantial evidence that Aduhelm reduces amyloid beta plaques in the brain and that the reduction in these plaques is reasonably likely to predict important benefits to patients. As a result of FDA’s approval of Aduhelm, patients with Alzheimer’s disease have an important and critical new treatment to help combat this disease. FDA will continue to monitor Aduhelm as it reaches the market and ultimately the patient’s bedside. Additionally, FDA is requiring Biogen to conduct a post-approval clinical trial to verify the drug’s clinical benefit. If the drug does not work as intended, we can take steps to remove it from the market. But hopefully, we will see further evidence of benefit in the clinical trial and as greater numbers of people receive Aduhelm. As an agency, we will also continue to work to foster drug development for this catastrophic disease. 文章來源:www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease?fbclid=IwAR2_dfZqAgfSkGGHOcLFtrZvjsTYuDvhB9kymPQr7C3y4pzi9vBLzw_XgRA |
依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。
恕不對外開放非專業人士使用。 每月文章
一月 2023
類別 |
營業時間:週一至週日(全年無休) 早上九點至晚上十一點四十分 (09:00~23:40)
|
地址:台北市松山區饒河街204號
|
聯絡我們 |

 RSS 訂閱
RSS 訂閱
