|
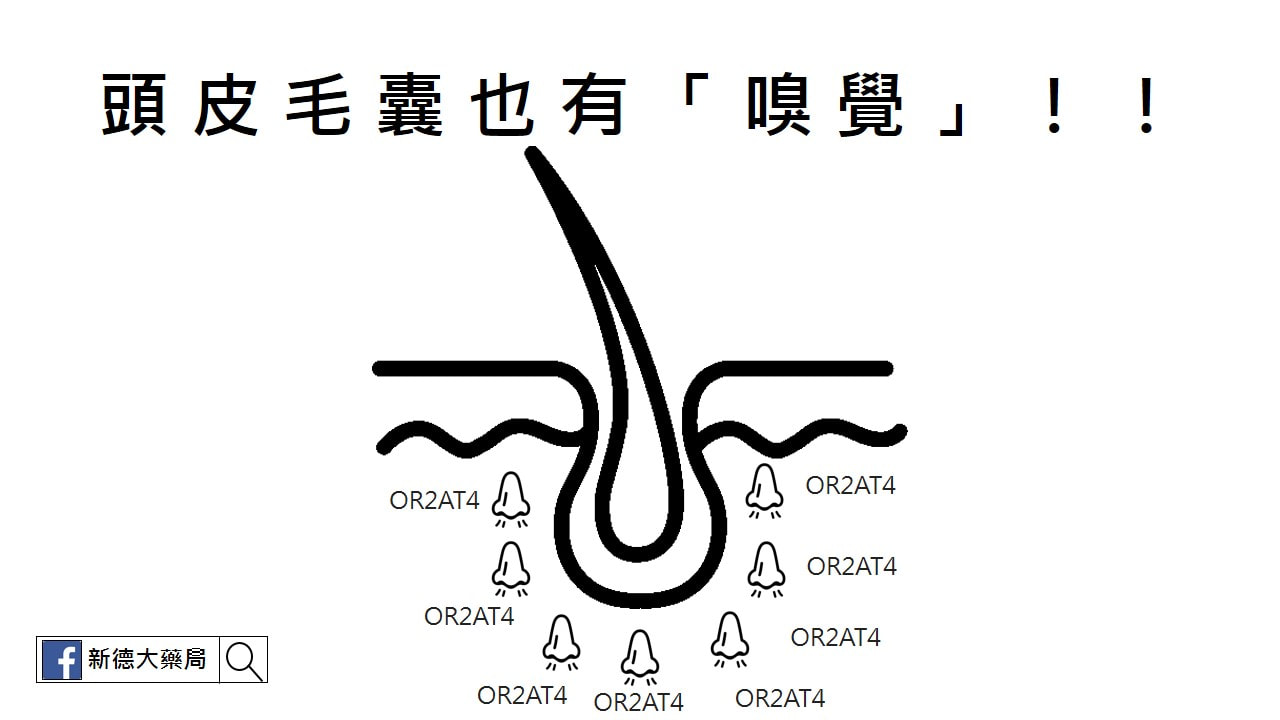
除了鼻腔裡的嗅覺細胞,近期一項國際知名NATURE的人體外試驗研究發現,頭皮毛囊也有「嗅覺」,若接受到特定香味的刺激,甚至能促使毛髮生長! Published: 18 September 2018 Olfactory receptor OR2AT4 regulates human hair growth 國曼徹斯特大學聯合美國、德國學者的研究指出一項驚人發現,人類毛囊細胞也有被稱為「OR2AT4」的嗅覺受體,可以聞到不同的氣味(結合氣味的化學分子,向大腦發出電子信號)。科學家至少發現了一種味道能刺激毛髮生長──這種味道正是常在芳香劑裡見到的「人工合成檀香味」。 研究團隊領導人,曼徹斯特大學的高級研究員兼皮膚科醫師Ralf Paus表示,「有史以來第一次證明,可以通過一種聞起來優雅又廣泛使用的芳香劑,來促進人體毛髮的生長。」 為了進行這個實驗,研究人員用了整形手術的患者捐贈的頭皮樣本,在實驗環境下使用人工合成檀香Sandalore刺激這塊頭皮,隨後毛囊細胞中一種「種類胰島素生長因子IGF-1」分泌提高,而這種激素能促進毛髮生長。 將另一種聞起來帶有花香甜味的人工香味劑Phenirat與毛囊細胞接觸後,IGF-1的分泌反而產生了抑制。這說明毛囊擁有自己「喜歡與否」的氣味,而反應在毛髮生長的情況上。 目前研究團隊認為用香味治療禿頭的方式大有可為,明年一月將啟動大型人體臨床實驗。 在沒有證實之前,建議還是先使用正規的醫療方式,需要使用外用生髮液(落建)或是口服用藥(柔沛或是新髮靈),都還是得照原來的方式。 千萬不要因為這個新聞,就停止用藥,改買人工合成檀香味的芳香劑來薰頭皮毛囊,萬一方法有誤,可能導致更嚴重的落髮喔! AbstractOlfactory receptors are expressed by different cell types throughout the body and regulate physiological cell functions beyond olfaction. In particular, the olfactory receptor OR2AT4 has been shown to stimulate keratinocyte proliferation in the skin. Here, we show that the epithelium of human hair follicles, particularly the outer root sheath, expresses OR2AT4, and that specific stimulation of OR2AT4 by a synthetic sandalwood odorant (Sandalore®) prolongs human hair growth ex vivo by decreasing apoptosis and increasing production of the anagen-prolonging growth factor IGF-1. In contrast, co-administration of the specific OR2AT4 antagonist Phenirat® and silencing of OR2AT4 inhibit hair growth. Together, our study identifies that human hair follicles can engage in olfactory receptor-dependent chemosensation and require OR2AT4-mediated signaling to sustain their growth, suggesting that olfactory receptors may serve as a target in hair loss therapy.
IntroductionOlfactory receptors (ORs) are part of an evolutionarily ancient chemosensory signaling system that long predates the development of smell sensation (olfaction)1,2,3,4,5,6,7. OR expression is not restricted to the nasal epithelium, but it is also present in several other human tissues8,9,10,11,12,13,14,15. Non-olfactory roles of ORs have also been described in human cell physiology16, such as in spermatozoa10,17 and enterochromaffin cells of the gut18. Interestingly, several ORs are also expressed in human epidermis19,20, including OR2AT4, whose selective activation by the synthetic sandalwood odorant (Sandalore®) promotes human epidermal keratinocyte migration and proliferation in vitro and wound re-epithelialization ex vivo20. Sandalore®-induced Ca2+ signaling could be blocked in OR2AT4-transfected Hana3A cells when this was co-applied at equimolar concentrations with the potent competitive OR2AT4 antagonist in presence of Sandalore®, Phenirat®20. Given the intimate connections between hair growth and wound healing21,22,23,24, we hypothesized that this OR might also impact on human hair growth. This hypothesis was investigated by immunohistology, qRT-PCR, western blot, microarray, phospho-kinase assay, and gene silencing in healthy, organ-cultured human scalp hair follicles (HFs)25. The present study shows that human HFs express a specific OR, namely, OR2AT4. The activation of this OR by its specific agonist, Sandalore®, prolongs anagen maintenance ex vivo by decreasing hair matrix keratinocytes apoptosis and increasing the production of IGF-1 in the outer root sheath (ORS). The anagen-prolonging effect mediated by Sandalore® is OR2AT4 dependent, as confirmed by co-administration of Sandalore® with the OR2AT4 competitive antagonist, Phenirat®, as well as the specific knock-down of OR2AT4 in human HFs. Taken together, we show that human HFs can engage in chemosensation and that the specific activation of OR2AT4 is required to sustain HF growth. ResultsHuman HFs express OR2AT4Immunofluorescence microscopy, qRT-PCR, and western blot analysis revealed that human scalp HFs in the anagen VI stage of the hair cycle26,27 express OR2AT4 at the transcript and protein level (Figs. 1a, 2a–f). Interestingly, OR2AT4 protein was predominantly expressed by suprabulbar keratinocytes of the proximal ORS (Figs. 1a, 2c), while hair matrix keratinocytes also expressed low-level OR2AT4 protein (Figs. 1a, 2b), both in healthy scalp skin in situ27 (Fig. 2a–c) and in amputated microdissected anagen HFs ex vivo25,26 (Fig. 1a). Of note, OR2AT4 expression was downregulated during spontaneous, apoptosis-driven HF regression (catagen)26,27 (Fig. 2d–g). Thus, using the primary antibody employed here20, intrafollicular OR2AT4 expression is strikingly restricted to defined epithelial HF compartments and is hair cycle dependent. Fig. 1 Hair follicles express OR2AT4, which specific stimulation endorses IGF-1-dependent anagen prolongation. a Representative images showing OR2AT4 protein expression (using the previously published OR2AT4 antibody20) in proximal outer root sheath and hair matrix keratinocytes of human scalp microdissected hair follicles. b Hair cycle score and staging were evaluated in treated and vehicle HFs after 6 days of culture using Ki-67/TUNEL immunofluorescence and Masson–Fontana histochemistry26. Mean ± SEM, n = 16–24 HFs from three donors (independent experiments), Kruskal–Wallis test (P = 0.0923, n.s not significant) and Dunn’s multiple comparisons test as post hoc test, ns not significant, Mann–Whitney test, *P < 0.05. Representative pictures of Masson–Fontana histochemistry in vehicle and treated HFs after 6 days of treatment. c Apoptotic hair matrix keratinocytes were counted in the hair matrix of all treated and vehicle HFs. Representative pictures of Ki67/TUNEL. Mean ± SEM, n = 18–21 HFs from three donors (independent experiments), Kruskal–Wallis (P = 0.005) test and Dunn’s multiple comparisons test as post hoc test, #P < 0.05, ##P < 0.01, ###P < 0.001. d IGF-1 expression was measured in ORS keratinocytes in treated and vehicle HFs. Representative pictures of IGF-1 immunofluorescence. IGF-1 expression was quantified in ORS keratinocytes in treated and vehicle HFs using ImageJ. Mean ± SEM, n = 18–21 HFs from three donors (independent experiments), Kruskal–Wallis (P < 0.001) and Dunn’s multiple comparisons test as post hoc test, ##P < 0.01, ###P < 0.001, and Student’s t-test, *P < 0.05. e Hair cycle score and staging were measured in treated and vehicle HFs after 6 days of culture. Representative pictures of vehicle and treated HFs after 6 days of treatment. Mean ± SEM, n = 22–29 HFs from three donors (independent experiments), Kruskal–Wallis test (P = 0.1434) and Dunn’s multiple comparisons test as post hoc test, n.s not significant, and Student’s t-test after performing an iterative Grubbs outlier test, *P < 0.05. CTS connective tissue sheath, DP dermal papilla, HM hair matrix, ORS outer root sheath, IRS inner root sheath, HS hair shaft. Scale bar: 100 µm Full size image Fig. 2 OR2AT4 mRNA and protein expression in human scalp epidermis and hair follicles in situ. aRepresentative pictures of OR2AT4 immunofluorescence in human scalp epidermis of three different donors (positive control20). Red line delineates the dermo-epidermal basement membrane. Cytosolic expression of OR2AT4 in hair matrix and suprabulbar outer root sheath (ORS) keratinocytes. Scale bar: 100 µm. b, c Representative pictures of confocal imaging of OR2AT4 immunofluorescence in human scalp HFs from three different donors (independent experiments). Scale bar: 100 µm. d, e mRNA (normalized against GAPDH) and in situ protein expression of OR2AT4 in anagen and catagen microdissected HF epithelium. Mean ± SEM, n = 3 from nine HFs/donor from three donors (independent experiments), Student’s t-test, *P < 0.05. f, g Western blot analysis and quantitative results of OR2AT4 (normalized against actin) in anagen and catagen microdissected human scalp HFs. Mean ± SEM from nine HFs/donor from two donors (independent experiments). The specific band for OR2AT4 is found around 44 kDa, although the predicted molecular weight of OR2AT4 is 36 kDa. This slight difference can be explained by a post-translational modification (an acetylation site has been identified on the lysine at the position 303 [source: phosphoSitePlus®]) that would increase the molecular weight64. A1-2 indicates anagen HFs and C1-2 indicates catagen HFs from donor 1 and 2. DP dermal papilla, HM hair matrix Full size imageOR2AT4 activation by Sandalore® prolongs anagen ex vivoWhen microdissected, organ-cultured human HFs25 were treated with Sandalore® (500 μM, for details, see Supplementary Note 1 and Fig. 3a–e) for 6 days, this selective OR2AT4 agonist20 significantly upregulated intrafollicular OR2AT4 protein expression (Fig. 3d), demonstrating receptor functionality and that OR2AT4 expression underlies a positive feedback regulation. Fig. 3 High concentration of Sandalore® (500 µM) regulates hair matrix keratinocytes apoptosis and intrafollicular OR2AT4 expression. a, b The number of Ki-67+ and TUNEL+ cells in the hair matrix was evaluated in the hair bulb of all treated and vehicle HFs. c Representative pictures of Ki-67/TUNEL staining. Mean ± SEM, n = 19–21 HFs from two donors (independent experiments), unpaired Student’s t-test or Mann–Whitney test, *P < 0.05, **P < 0.01. DP dermal papilla, HM hair matrix. Scale bar: 100 µm. d OR2AT4 protein expression was evaluated using ImageJ in the ORS of Sandalore®-treated and control HFs after 6 days of culture. e Representative pictures of OR2AT4 expression in the ORS of cultured HFs. Mean ± SEM, n = 12–15 HFs from two donors (independent experiments), Student’s t-test, *P < 0.05. CTS connective tissue sheath, IRS inner root sheath, ORS outer root sheath. Scale bar: 100 µm Full size imageImportantly, Sandalore® treatment retarded spontaneous HF regression (catagen development)26,27 ex vivo (Fig. 1b) and significantly reduced hair matrix keratinocyte apoptosis, as shown by quantitative (immuno-)histomorphometry for TUNEL+ (Fig. 1c) or cleaved caspase 3+ cells (Supplementary Fig. 1a) in the hair matrix. These effects were partially counteracted by co-administering the competitive OR2AT4 antagonist, Phenirat®20, with Sandalore® (Fig. 1b, c, Supplementary Fig. 1a). When tested alone, Phenirat® tended to be weakly hair growth inhibitory (Supplementary Fig. 2a, b and Supplementary Note 2 for extended discussion). Next, we examined two key growth factors that control the anagen-catagen transformation during human HF cycling, i.e., catagen-promoting TGF-β2 and anagen-maintaining IGF-1; these growth factors are prominently produced by those proximal ORS keratinocytes28,29,30,31,32,33 that express OR2AT4 maximally. This analysis revealed a significant decrease in TGF-β2 (Supplementary Fig. 3a) and a significant increase of IGF-1 (Fig. 1d) protein expression in the proximal ORS after long-term Sandalore® treatment ex vivo. The co-administration of OR2AT4 antagonist, Phenirat®, significantly reversed the Sandalore®-induced intrafollicular upregulation of IGF-1 (Fig. 1d) but did not affect TGF-β2 expression (Supplementary Fig. 3a). Anagen-prolonging effect of Sandalore® is OR2AT4 specificSubsequently, we selectively silenced OR2AT4 by siRNA administration to organ-cultured human scalp HFs ex vivo32,34,35, as documented by significantly reduced intrafollicular OR2AT4 mRNA and protein expression (Fig. 4a, b). Despite the presence of excess ligand (Sandalore®), OR2AT4 knock-down significantly promoted catagen induction compared to HFs treated with scrambled oligos (Fig. 5a), decreased IGF-1 protein expression (Fig. 5b), and enhanced hair matrix keratinocyte apoptosis (Fig. 5d, e). Instead, hair matrix keratinocyte proliferation (Fig. 5c) or TGFβ2 protein expression in the ORS (Supplementary Fig. 4a) remained unaffected. These data show that the Sandalore®-induced hair growth stimulation documented above is indeed OR2AT4 dependent, rather than due to off-target effects of this synthetic odorant and that OR2AT4 signaling is required for anagen maintenance. Fig. 4 OR2AT4 expression in Scambled oligos or siRNA OR2AT4 Sandalore®-treated, microdissected human scalp HFs after 6 h of organ culture. a Microdissected, human scalp HFs were treated for 6 h with OR2AT4 siRNA or scrambled oligos, using the intrafollicular gene silencing technique32. OR2AT4 mRNA expression was evaluated after 24 h of culture in siRNA or scrambled oligo-transfected HFs. b OR2AT4 protein expression was evaluated using ImageJ in the ORS of siRNA-treated and control HFs after 6 days of culture. Representative pictures of OR2AT4 expression in the ORS of cultured HFs. Mean ± SEM, n = 19–24 HFs from three donors (independent experiments), Student’s t-test, *P < 0.05, ***P < 0.001. CTS connective tissue sheath, IRS inner root sheath, ORS outer root sheath. Scale bar: 100 µm Full size image Fig. 5 The anagen prolongation effect of Sandalore® is OR2AT4 dependent. a Hair follicle cycle score and staging were performed in HFs treated with siRNA OR2AT4 or scrambled oligos in the presence of Sandalore®32. Representative images of vehicle and treated HFs after 6 days of treatment. Mean ± SEM, n = 16–18 HFs from three donors (independent experiments), Mann–Whitney test, *P < 0.05. b IGF-1 expression was quantified in ORS keratinocytes in treated and vehicle HFs using ImageJ. Representative pictures of IGF-1 immunofluorescence. Mean ± SEM, n = 22–24 HFs from three donors (independent experiments), Mann–Whitney test, *P < 0.05. c, d Ki67+ cells and TUNEL+ cells were counted in the hair matrix of siRNA and control HFs. Representative pictures of Ki67/TUNEL double-staining in the hair bulb of HFs. Mean ± SEM, n = 17–18 HFs from three donors (independent experiments), Mann–Whitney test, *P < 0.05, n.s. not significant. e The number of cleaved caspase-3+ cells (white arrows) in the hair matrix was evaluated in the hair bulb of all HFs treated with OR2AT4-siRNA or scrambled oligos. Representative pictures of cleaved caspase-3 staining. Mean ± SEM, n = 18 HFs from three donors (independent experiments), Student’s t-test, *P < 0.05. CTS connective tissue sheath, DP dermal papilla, HM hair matrix, ORS outer root sheath, IRS inner root sheath. Scale bar: 100 µm Full size imageSandalore®-mediated HF response involves different pathwaysMicroarray analysis independently confirmed anti-apoptotic effects of Sandalore® (Fig. 6, Supplementary Fig. 5, and Supplementary Data 1), since transcripts of pro-apoptotic genes were significantly downregulated (e.g., TP53AIP1: -10.27×), while anti-apoptotic genes were significantly upregulated (e.g., FGF-2: +7.83×) in HFs treated short term with Sandalore® (6 h, Supplementary Fig. 5a, b and Supplementary Data 1). Interestingly, an additional microarray analysis of organ-cultured scalp HFs in which OR2AT4 had been knocked down ex vivo showed that transcription of the IFI6 (G1P3) gene, whose silencing increases keratinocyte apoptosis36, was downregulated by administering OR2AT4 siRNA for 6 h, compared to scrambled oligonucleotide-treated HFs (Supplementary Fig. 6a, b and Supplementary Data 1). This corresponds well to our observation that OR2AT4 silencing increases apoptosis of HF matrix keratinocytes (Fig. 5d, e) and further underscores the importance of continued OR2AT4 stimulation by as yet unknown endogenous ligands to suppress apoptosis in the hair matrix of human anagen HFs. Fig. 6 Microarray-based analysis of genes related to anagen-prolonging pathways after stimulation with Sandalore® (500 µM). a, b Venny diagrams65 show the upregulated and downregulated genes (cut-off: fold change >−1.8 or >+1.8 and equidirectional changes). White squares indicate genes upregulated and downregulated in at least three of four donors (independent experiments). The heatmap shows the list and the expression level of the most upregulated and downregulated genes related to the different pathways regulated after OR2AT4 activation (cut-off: fold change >−1.8 or >+1.8 and equidirectional changes) in at least three of four donors (independent experiments) (c). Green: apoptosis related, orange: dermcidin related, and violet: IGF related Full size imageIn addition, microarray analysis revealed that Sandalore® promotes signaling along the IGF pathway (see Fig. 6a–c, Supplementary Fig. 5a-b, and Supplementary Data 1), in agreement with the protein expression data (Fig. 1d). Indeed, genes involved in IGF1R signaling cascade as well as in IGF transport (e.g., PAPPA [10.8× upregulated] that cleaves IGFBP4 to release IGF37, or PCSK-1 [33.6× upregulated] which is involved in insulin synthesis from proinsulin38) were strongly upregulated (Fig. 6a–c, Supplementary Fig. 5, and Supplementary Data 1). This promotion of IGF signaling pathway as well as the upregulation of FGF-7 (2.75× increase), another anagen-promoting growth factor39 (Fig. 6a–c, Supplementary Fig. 5, and Supplementary Data 1) are perfectly in line with anagen prolongation by Sandalore®. Intriguingly, the strongest transcriptional upregulation (77.6× increase) was seen for dermcidin, a potent antimicrobial peptide with broad bactericidal activities that reportedly is only produced by sweat gland epithelium in human skin40,41. However, quantitative immunohistomorphometry confirmed that dermcidin protein is also upregulated by Sandalore® in the epithelium of human scalp HFs (Supplementary Fig. 7a and Table 1). This demonstrates that human HFs also express dermcidin and raises the fascinating question whether OR2AT4 may act as a chemosensory receptor for selected bacterial metabolites, in response to which intrafollicular dermcidin production may be upregulated to manage the complex HF-microbiome42,43. Table 1 OR2AT4 stimulation by Sandalore®treatment regulates different signaling protein phosphorylation pathways in human HFs ex vivo Full size tableWhen selected signaling pathways recognized to be involved in OR-mediated signaling1,9,44,45,46,47, were studied by phospho-kinase assay9, Sandalore® upregulated several expected kinase activities (Fig. 7a and Table 1). In line with our previous results (Fig. 1c, d), this included increased phosphorylation of proline-rich AKT1 substrate 40 (PRAS40), whose expression is induced by IGF-148,49, while Sandalore® reduced phosphorylation of p53 (S46), which is highly phosphorylated in apoptotic cells50 (IGF-1 is a key apoptosis suppressor51,52,53). Fig. 7 OR2AT4 stimulation by Sandalore® treatment regulates different protein phosphorylation signaling pathways in human HFs ex vivo. Plot showing regulation of the phosphorylation of 45 kinases mediated by Sandalore® (50 and 500 µM) in cultured microdissected HFs from four different donors (independent experiments). Among those kinases, the most interesting ones underline in green (upregulated) and red (downregulated) rectangles. PRAS40 proline-rich AKT1 substrate 4049, S46 phosphorylated p5350, p53 phosphoprotein p53, p38α mitogen-activated protein kinases 14, ERK1/2 extracellular signal-regulated kinases1/2, EGFR epidermal growth factor receptor, MSK1/2 mitogen- and stress-activated protein kinase 1/2, PYK2 protein tyrosine kinase 2, Hsp60 heat shock protein 60, JNK1/2/3 c-Jun N-Terminal Protein Kinase 1/2/3, AMPKα1 AMP-activated protein kinase α1, PLCγ phospholipase C γ1, Fgr Feline Gardner-Rasheed proto-oncogene, WNK1 WNK lysine-deficient protein kinase 1 isoform Full size imageAnagen-prolonging effect of Sandalore® implicates IGF-1Therefore, we next probed the hypothesis that, mechanistically, Sandalore® stimulation of OR2AT4 may retard catagen and suppress HF apoptosis by upregulating intrafollicular IGF-1-mediated signaling. Indeed, the co-administration of IGF1-neutralizing antibody with Sandalore® significantly reversed the catagen-promoting effect of IGF-1 neutralizing antibody alone (Fig. 1e; for extended discussion, see Supplementary Note 3). Mechanistically, this suggests that OR2AT4 activation mainly prolongs anagen via upregulating IGF-1 expression and secretion by OR2AT4+ keratinocytes in the proximal ORS (Figs. 1d, 8). While IGF-1 signaling is known to be involved in olfactory bulb development and function54,55,56, the current study reveals that IGF-1 expression/secretion in human epithelial tissue is also controlled by OR-mediated signaling and demonstrates that IGF-1 production underlies an OR2AT4-controlled chemosensory regulation. Fig. 8 Proposed mechanism of action of OR2AT4 activation by Sandalore® and (unknown) endogenous ligand(s) in human hair follicle epithelium. The activation of OR2AT4 at the cell surface of outer root sheath keratinocytes (ORS KCs; location: see green cells in the central HF cartoon) by endogenous ligands and/or Sandalore® upregulates the expression of genes and kinases involved in programmed cell death, thus preventing intrafollicular apoptosis (e.g., by phosphorylation of PRAS40 preventing its interaction with mTOR1, upregulation of NF-κB pathway) or downregulates key players in the apoptotic machinery (e.g., dephosphorylation of p53, downregulation of Bad). In parallel, OR2AT4 activation by exogenous (Sandalore®) or endogenous ligands (e.g., metabolites of the HF microbiome) induces the upregulation of PAPPA that cleave the IGFBP4/IGF1 complex to release IGF-1 (pink arrows). The released IGF-1 triggers the activation of IGF-1R on the same cell (autocrine signaling, purple arrow) or on hair matrix keratinocytes (HM KCs; orange “cell”) (paracrine signaling, orange arrow). The activation of IGF-Rs on HM keratinocytes then induces signaling cascades (e.g., PI3K/AKT and/or p38a/ERK1/2/MSK1/2) that activate different transcription factors and particularly CREB, which results in an anti-apoptotic effect and prolonged anagen phase in human HFs. P phosphorylation, green square gene upregulation, red square gene downregulation, green circle phosphorylation, red circle dephosphorylation Full size imageDiscussionCollectively, these data show that the growth, cyclic transformation, epithelial cell apoptosis, and IGF-1 production of a dynamic human (mini-)organ, i.e., scalp HFs29, underlies an OR-dependent chemosensory control. Thus, human HFs can “smell” in the sense that they recruit the evolutionarily oldest and largest of all receptor families1,2 for regulating key organ functions (for extended discussion, see Supplementary Discussion 1). Moreover, we identify one specific OR, namely, OR2AT4, whose stimulation with a synthetic agonist (Sandalore®)20 and whose selective silencing profoundly impacts on human hair growth ex vivo primarily via regulating expression and secretion of the key hair growth-promoting factor, IGF-1 (Fig. 8 and Supplementary Discussion 2). However, while IGF-1-mediated signaling is required for human hair growth promotion by Sandalore® (Fig. 1e), our phospho-kinase activity and gene expression profiling results suggest that additional pathways (e.g., p38a/ERK1/2/MSK1/2, HB-EGF/EGF-R, and FGF-7 pathways (Fig. 6, Supplementary Figs. 5, 6, and Supplementary Data 1)) are involved that deserve further exploration57,58,59,60,61. Perhaps most intriguingly, our silencing data suggest that OR2AT4-mediated signaling is required for maintaining human scalp HFs in anagen and for suppressing keratinocyte apoptosis in the hair matrix (Fig. 5d, e). This begs the question: What are the endogenous intrafollicular OR2AT4 ligands in human HFs? The endogenous ligands for human ORs remain to be definitively clarified, and those for OR2AT4 are unknown. Candidates include molecules with Sandalore®-like structure, short-chain fatty acids13, and—namely, in view of our dermcidin results (Supplementary Fig. 7a and Table 1)—metabolites of resident HF microbiota42,43. Taken together, our ex vivo data suggest that olfactotherapy by topically applied cosmetic OR2AT4 ligands like Sandalore® may promote human hair growth by prolonging anagen and inhibiting premature catagen development (e.g., in androgenetic alopecia and telogen effluvium). Thus, using scalp HFs as accessible and tractable model organs and by selectively targeting OR2AT4, our study reveals an important, translationally relevant frontier in the OR-dependent chemosensory physiology of peripheral human tissues. MethodsHuman samplesTemporal and occipital human scalp skin was obtained from healthy donors (38–69 years old) undergoing routine face-lift surgery after informed consent and ethical approval (University of Muenster, no. 2015-602-f-S). No sample size calculation was performed. Number of three different donors was used due to the small availability of the tissue used in the study. This number of three was used in many previous studies, given statistical significance. Tissue specimensScalp skin samples were either cut into small pieces (4 mm), embedded into OCT, and frozen in liquid nitrogen, or processed for HF microdissection25. HF organ cultureHuman scalp samples were obtained 1 day after face-lifting procedure (i.e., after overnight transport from collaborating surgeons) and used at the same day for microdissecting human anagen VI scalp HFs. The HF microdissection technique employed for setting up the classical Philpott assay25,26,62 used in the current study, removes all perifollicular tissue with the sole exception of the HF’s dermal sheath, and thus does not contain any other skin appendage structures (e.g., eccrine gland elements)25. Microdissected human scalp HFs were cultured at 37 °C with 5% CO2 in a minimal media of William’s E media (WEM, Gibco, Life Technologies) supplemented with 2 mM of L-glutamine (Gibco), 10 ng/ml hydrocortisone (Sigma-Aldrich), 10 μg/ml insulin (Sigma-Aldrich), and 1% penicillin/streptomycin mix (Gibco)25,26,62. After microdissection, the HFs were first incubated in WEM for 24 h for re-equilibration. HFs after quality control (fully pigmented and presence in anagen VI phase) were randomly allocated to the different experimental groups. Chemical stimulation of human microdissected HFsAfter 24 h, WEM medium was replaced and HFs were treated with vehicle (0.1% DMSO), Sandalore® (50 and 500 µM; see Fig. 3 and Supplementary Note 1, Symrise), Phenirat® (in a ratio 1:1 to the agonist, Symrise), or Sandalore®+Phenirat® for 6 days for (immuno-)histology or 6 h for qRT-PCR. For the IGF-1 neutralizing antibody experiments, IGF-1 neutralizing antibody (1 µg/ml, ab9572, Abcam) was added 30 min before adding Sandalore® to the corresponding groups. Culture medium was replaced every second day and after 6 days. HFs were then embedded in cryomatrix (Fisher Scientific), and snap frozen in liquid nitrogen for (immuno-)histology. SiRNA transfection-knockdown OR2AT4 in organ-cultured HFsHuman anagen VI HFs were transfected using a commercial siRNA reagent system (Sc-45064, Santa Cruz) following the manufacturer’s instructions32,34,35. Briefly, stock solutions (10 µM) of siRNA OR2AT4 (gift from Prof. Hanns Hatt20) and siRNA control (scrambled oligo) were prepared using RNAse-free water. HF transfection was performed 24 h after microdissection for 6 h using either 100 mM OR2AT4 siRNA or control scramble siRNA. After 24 h of incubation with fresh WEM medium, HFs were collected per group in RNA later and stored at 4 °C for further RNA extraction and qRT-PCR analysis or immediately frozen in liquid nitrogen and stored at −80 °C for microarray analysis. Finally, fresh WEM medium was replaced every second day and after 5 days of culture, HFs were snap frozen in OCT for further quantitative (immuno-)histomorphometry analysis. HistologyFor histochemical visualization of melanin, Masson–Fontana staining was performed on frozen sections. Melanin was stained as brown dots26. ImmunofluorescenceOCT-embedded samples were sectioned (6 µm thickness for HF and 7 µm thickness for skin) with a Leica cryostat. For primary OR2AT420(custom designed rabbit polyclonal antibody generated against the C-terminus sequence of OR2AT4 (Eurogentec, Liège, Belgium)), or cleaved-caspase-3 (#9661, clone Asp175, Cell Signaling) antibodies staining, tissue cryosections were fixed in 4% paraformaldehyde, pre-incubated with 10% of goat serum (for OR2AT4) or 5% goat serum +0.3% Tritton X-100 (for cleaved-caspase 3) and incubated with the corresponding primary antibody at 4 °C overnight (1/100 for OR2AT4 and 1/400 for cleaved-caspase 3). Secondary antibody incubation was performed at RT for 45 min. Counterstaning with DAPI (1 µg/ml) was performed to visualize nuclei. Dermicidin protein was detected using tissue sections fixed in 4% paraformaldehyde, pre-incubated with 10% of goat serum, and incubated with a mouse anti-human Dermcidin antibody (Novus Biologicals, G-81, 1:200). Secondary antibody (Goat anti-mouse Alexa fluor 488) incubation was performed at room temperature for 45 min. Counterstaning with DAPI (1 µg/ml) was performed to visualize nuclei. For TGFβ2 (Sc-90, Santa Cruz) and IGF-1 (Sc-1422, clone G-17, Santa Cruz31,32), tissue cryosections were fixed in acetone and endogenous peroxidase activity was blocked with 3% of H2O2 (Merck Milipore). This step was followed by an avidin-biotin blocking step (SP2001, Vectorlabs) and a preincubation with TNB buffer (Tris HCl+NaCl+Casein). The corresponding primary antibody was incubated at 4 °C overnight (1/1000 for TGFβ2 and 1/250 for IGF-1). Secondary antibody incubation was performed at RT for 45 min before using the Tyramide signal amplification kit (NEL700001KT, Perkin Elmer). Counterstaning with DAPI was performed to visualize nuclei. To stain apoptotic and proliferating cells, we used the apoptag kit (Merck Milipore) following the manufacturer’s protocol followed by Ki-67 staining25,26,33,63. Primary antibody was incubated overnight (Ki-67, M7240 Clone: MIB-1, DAKO, 1/20) after the TdT-enzyme step. The secondary antibody was incubated for 45 min at RT after the fluorescent-labeled anti-Digoxigenin step of the apoptag kit. Counterstaning with DAPI was performed to visualize nuclei. Negative controls were performed by omitting the primary antibody. Images were taken using a Keyence fluorescence microscope BZ9100 (Osaka, Japan) maintaining a constant set exposure time throughout imaging for further analysis. Quantitative reverse transcriptase-PCRTotal RNA was isolated from whole microdissected HFs using RNeasy Mini Kit (Quiagen) following the manufacturer’s instructions described in the manufacturer’s protocol. RNA purity and concentrations were determined using the Nanodrop ND-1000 assay (Fisher Scientific). Reverse transcription of the RNA into cDNA was performed using the TetrocDNA Synthesis Kit (Bioline), according to the manufacturer’s instructions. RNA concentrations were adjusted between 50 to 500 nM for each sample set to allow further quantification comparison between samples and experiments after qRT-PCR. Controls were performed using the housekeeping gene GAPDH. Real-time quantitative polymerase chain reaction (qRT-PCR) was run in triplicate using TaqMan Fast Advanced Master Mix Product Insert and gene Expression Assay transcripts (Id: Hs01060665_g1 for ACTB, Hs02758991_g1 for GAPDH, and Hs02339277_s1 for OR2AT4, Applied Biosystem) on the qTower2.2 thermocycler. Real-time quantification plots and Ct values were collected and stored by the qPCRsoft2.1 software. The amount of the transcripts was normalized to those of the housekeeping gene using the ΔΔCT method using EXCEL. Whole-genome microarray analysisRNA isolation, sample processing, and microarray analyses (Agilent Technologies), as well as statistical evaluation, were performed by Arrows Biomedical GmbH (Muenster, Germany). Expressional alteration was considered to be significant only when ≥1.8-fold and equidirectional changes were observed in at least three of four patients (independent experiments). An additional analysis has been performed using 5-fold and equidirectional changes in the four different donors (independent experiments) in order to identify the top up and downregulated genes. Human phospho-kinase arrayIn order to gauge which signaling pathways are regulated by the specific stimulation of OR2AT4, we performed a phospho-kinase array9. Total protein was isolated from whole microdissected HFs using a specific buffer from the Human Phospho-Kinase Array (ARY003B, R&D System), following the manufacturer’s protocol. Briefly, protein extracts were diluted and incubated overnight with the Human Phospho-Kinase Array. The array was washed to remove unbound proteins, followed by incubation with a cocktail of biotinylated detection antibodies. Streptavidin-HRP and chemiluminescent detection reagents were applied, and a signal was produced at each capture spot corresponding to the amount of phosphorylated protein bound. Western blot analysisTotal protein was extracted from nine anagen and catagen microdissected human scalp HFs. Protein concentrations were determined using a Bradford assay (B6916, Sigma-Aldrich). Thirty micrograms of protein were subjected to 4–15% Mini-PROTEAN®TGX™ Precast gel (#4561083, Bio-Rad) and transferred to a nitrocellulose membrane (88018, Thermo Fisher Scientific), followed by incubation with the corresponding primary antibodies (PA5-71599 for OR2AT4, 1/1000, Thermo Fisher Scientific; and A3853 for Actin, 1/1000, Sigma-Aldrich) overnight at 4 °C. After incubation with peroxidase-conjugated secondary antibodies (WesternBreeze™ Chemiluminescent Kit, WB7106 and WB7104, Thermo Fisher Scientific), the bands were visualized using Chemocam imager 6.0 (Intas, Germany). Protein expression levels were normalized to corresponding actin levels. The uncropped blots are presented in the Supplementary Fig. 8. Hair cycle score (HCS) and stagingHFs were microscopically evaluated for the hair cycle staging analysis using Masson–Fontana histochemistry and Ki-67/TUNEL immunostainings25,26. The HCS was also measured25,31, which consists of assigning an arbitrary unit for each stage of the hair cycle (Anagen VI = 100; Early catagen = 200; Mid-catagen = 300; and Late catagen = 400). After having classified each HF according to its hair cycle stage, following the previously defined objective classification criteria for organ-cultured human HFs26, for each experimental condition, the mean HCS was calculated. The closer the mean is to 100, the higher is the number of anagen VI HFs in a given group. The HCS provides a global read-out parameter that looks at all HFs in a given experimental group and synthesizes them into a single number, which reflects how close the majority of HFs is to either anagen VI or catagen and also permits statistical analysis that it is not possible with hair cycle staging. Therefore, hair cycle staging and the HCS are independent read-out parameters that complement each other. Quantitative (immuno-)histomorphometryStaining intensity was evaluated in well-defined reference areas by quantitative (immuno-)histomorphometry 31,32, using NIH ImageJ software (NIH, Bethesda, MD, USA). Statistical analysesAll data are expressed as mean ± SEM (and variance is different between the groups) and were analyzed by one-way ANOVA or Kruskall–Wallis test and Dunn’s multiple comparisons test as post hoc test when more than two groups were compared or Student’s t-test or Mann–Whitney test when only two groups were compared (GraphPad Prism 6, GraphPad Software, San Diego, CA, USA) after performing d’Agostino and Pearson omnibus normality test. P < 0.05 was regarded as significant.
0 評論
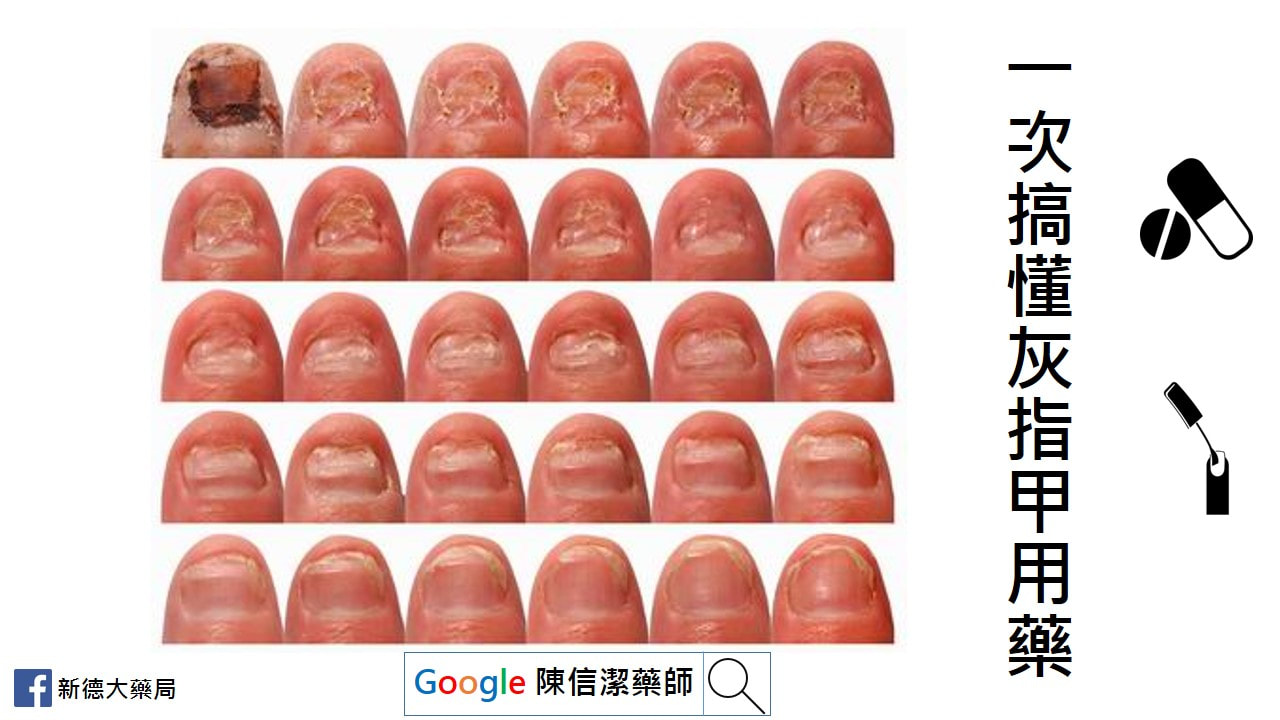
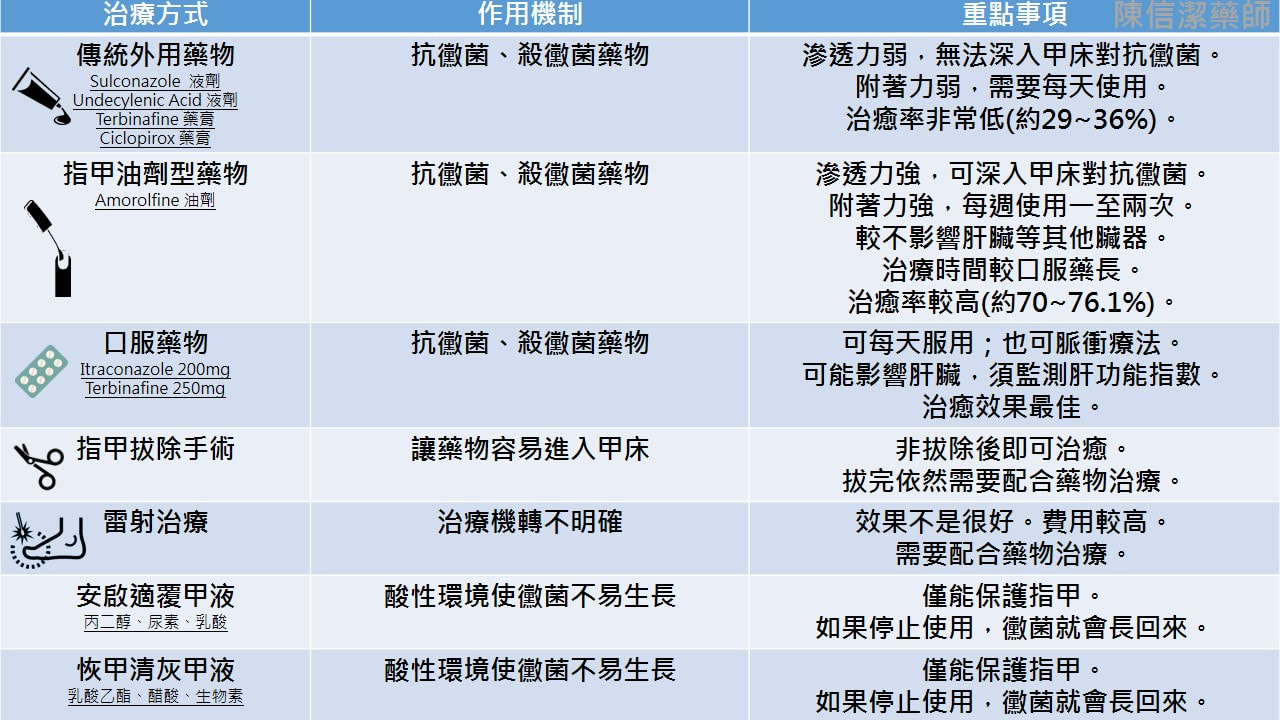
灰指甲就是指甲受黴菌感染,灰指甲常會併發在香港腳(足癬)患者身上。 正式的名稱叫『甲癬』。 台語稱『臭甲』。 哪些人容易感染灰指甲? 1、老年人 2、赤腳走在游泳池、健身房的淋浴間、公共浴池…等潮濕的表面 3、香港腳(足癬)患者 4、足部容易有小傷口,或是其他皮膚疾病,例如乾癬患者 5、糖尿病患者 6、各種免疫缺乏疾病患者 7、家中有其他人罹患灰指甲 灰指甲一定是灰色的嗎? 灰指甲常見的顏色有黃色、褐色、綠色等。 為什麼建議治療灰指甲? 嚴重者會有指甲增厚、周邊脫屑、指甲凹陷、指甲變形、甚至是甲床分離的情形。 有時會導致甲溝炎、凍甲、鉗甲等。 更嚴重者,還需要指甲矯正。 灰指甲合併香港腳會? 跟香港腳互相交錯感染,嚴重感染時,甚至偶會導致蜂窩性組織炎。 灰指甲會傳染嗎? 只要鞋子放在一起,或是一同出入潮濕地面,都很容易傳染。 例如:父母有灰指甲就很容易傳染給小孩。 治療灰指甲的方法有 1、傳統外用藥物 2、指甲油劑型藥物 3、口服藥物 4、指甲拔除術 5、雷射治療 安啟適是甚麼呢? 在他們的官網上找到一篇關於此產品的研究文獻(JCDSA, Vol.1, No.3, 2011, pp.59-63),讓我們來一探究竟: 文獻中提到安啟適®覆甲液的成分為丙二醇、尿素和乳酸。 丙二醇是常見於化妝品中的成分,可做為乳化劑及防腐劑,美國FDA歸類為「大致認為安全食品」的食品添加物。 尿素通常在藥品或化妝品中用於保濕及去角質,幫助藥品吸收滲透。 乳酸也可用於軟化角質。 此三成分用於灰指甲的配方在一篇1989年的文獻中提出,因此可說是老藥新賣吧。 官網提供的文獻是在瑞典進行,收案75人,最終72人完成整個療程。值得一提的是,實驗中將近端甲下真菌病(proximal subungual onychomycosis,PSO)排除,也許是因為此類灰指甲較常是因糖尿病、愛滋病等其他疾病引起吧。 實驗在第2、4、8周各追蹤一次,最終第8周病人在四級自我評估結果大多落在中間「部分改善」(39.7%)與「明顯改善」(39.7%),8.2%的人認為「無感善」,12.3%認為「非常好的改善」。 「安啟適®覆甲液」算是一個老配方新產品,文獻的實驗規模不大,單就實驗結果來看,使用「安啟適®覆甲液」後8周多數患者感覺灰指甲狀況有改善,但完全治癒的比率並不高。以安全性而言此配方式算是安全且少有副作用的成分。 恢甲清又是什麼呢? 成分有乳酸乙酯(乳化劑及防腐劑)、 醋酸<獨家突破性滲透技術(TransActive™Technology)>沒有實驗證明,也沒有專利? 、生物素(提供指甲營養素....靠外用的吸收營養恐怕有點難度) 不論恢甲清或是安啟適在台灣此產品申請為醫材,效能是「限保護因真菌感染與創傷而受損的指甲」,和廣告中宣稱的改善灰指甲感染問題有微妙的差異..... 治療灰指甲還是要以正 藥物治療為主,至於恢甲清或是安啟適則是用來改善外觀,無法達到指甲上完全沒有黴菌的效果。因此如果停止使用,黴菌就又長回來。 食品藥物管理署(下稱食藥署)檢出「"溫士頓" 好視多眼用懸浮液1公絲/公撮 FOXONE OPHTHALMIC SUSPENSION 1MG/ML "WINSTON" (衛署藥製字第044167號)」(批號 FX-17019)藥品含有未核准添加之Tropicamide散瞳劑成分藥品,食藥署已於9月13日要求業者啟動回收作業,通知醫療機構及藥局立即下架停止供應,並於1個月內(107年10月13日前)完成回收,請衛生局督導下架回收事宜。請民眾立即停用案內藥品,並立即回診。
我國核准「"溫士頓" 好視多眼用懸浮液1公絲/公撮FOXONE OPHTHALMIC SUSPENSION 1MG/ML "WINSTON" (衛署藥製字第044167號)」藥品,許可證持有廠商及製造廠為溫士頓醫藥股份有限公司,適應症為眼瞼炎、結膜炎、角膜炎、強膜炎、上強膜炎、虹彩炎、虹彩毛樣體炎。此次係檢出Tropicamide成分,該成分屬於散瞳劑,一般使用於眼科檢查及假性近視,可能短暫發生畏光、視力模糊等情形。 為確保藥物安全與醫療效能,食藥署已建置藥物安全監測機制,即時監視國內、外藥物安全訊息,除設有藥物不良反應通報系統及藥物不良品通報中心之外,並對於藥物之安全性與療效亦隨時進行再評估,如醫療人員或病患疑似因使用(服用)藥品導致不良反應之發生,請立即通報衛生福利部所建置之全國藥物不良反應通報中心,藥物不良反應通報專線02-2396-0100,網站:https://adr.fda.gov.tw。如發現藥物不良品時,請立即通報衛生福利部所建置之全國藥物不良品通報中心,藥物不良品通報專線02-6625-1166分機6401,網站: https://qms.fda.gov.tw。 首先,要了解自己一天有本錢吃多少熱量(就是俗稱的每日新陳代謝)。
在減重第一步中,教大家基礎代謝的計算方式,因為基礎代謝約佔一天所消耗熱量的七成,所以只要將基礎代謝除以0.7就可以估算出一天的新陳代謝有多少。 #減重第一步 那有沒有什麼方法可以增加自己的新陳代謝呢? 在臨床上,這是最多減重者問的問題。答案是當然有。下面我將就新陳代謝的角度來分析一些可增加新陳代謝的方法。 新陳代謝主要由三個方面決定,分別是: 一、 基礎代謝(佔約70%) 二、生理活動量(佔約20%) 三、攝食產熱效應(佔約10%) 因此,可從在這三個方面努力來增加整體的新陳代謝。 【一、基礎代謝方面】 所謂的基礎代謝就是維持生理機本活動所需的熱量,例如呼吸、心跳、血液循環、體溫維持等,這是維持生命所需的最基本熱量。在基礎代謝中,主要是由肌肉組織所負責,其次是肝臟、腸胃、腎臟、脾臟、心臟及腦等其他代謝性的器官,如果能夠維持這些器官組織的機能在最旺盛的狀態下,就能讓身體消耗最多的熱量。 而提供這些活性代謝組織所需的營養與能源就是維持這些組織機能的最好方法。 簡單地說,就是多吃營養的食物。 要維持良好的新陳代謝,你必須提供身體營養的食物,而不是只注重在卡路里上(此即營養減肥的原理)。這就好比要讓一台車子效能發揮到最好,除了汽油(熱量)外,潤滑油或機油(維生素與礦物質等營養素)也是不可或缺的,短時間也許看不出來,長時間絕對會有差異。臨床上,常看到很多人想減肥的人只專注在卡路里上,為了減肥常常吃固定缺乏營養與變化的食物,例如每天只以吐司與牛奶為當一餐,或吃蘋果餐,或只吃某些特定的東西,這種只重熱量不管營養的減肥方式,一開始體重或許會瘦,但最後常影響身體整體機能,使新陳代謝降低,導致最後只能越吃越少,且一但停止少吃減肥,體重很快就復胖回去。 提升基礎代謝的方法 簡單地說,就是吃營養價值高的食物。一樣300大卡,三明治等有肉有菜的食物優於蛋糕或奶茶等營養價值低的食物。 1、用胚芽米、五穀飯或糙米飯來取代白米飯。 2、用全麥、黑麥或五穀麵包(吐司)取代白麵包或白吐司。 3、以陽春麵搭配一顆蛋或一盤豆乾來取代麻醬麵、涼麵或乾麵。 4、以飯+烤或滷或蒸肉類+菜的均衡組合來取代炒飯、炒麵、義大利奶油麵等單一組合。 5、不要用單一食物減肥法減肥(如只吃蘋果,只喝蔬菜湯,只吃肉類或其他缺乏變化的減肥食物)。 6、每餐有飯有肉有菜,不要以吐司,蘇打餅乾、水果或代餐來取代正餐。 (註:減重代餐一天只建議吃一次) 【二、生理活動方面】 所謂的生理活動包括一般活動(如走路,爬樓梯,做家事),以及我們俗稱的跑步,游泳與運動。雖然一般生理活動每小時所消耗的熱量較少,但因能持續較久的時間,所以累績下來所消耗的熱量有時反而會比從事運動高。舉例來說,慢跑所消耗的熱量雖然比快走多,但慢跑無法持續太久。 但快走卻很容易持續數十分到數小時的時間(相信很多女性同胞都有以”時”計的壓馬路習慣)。 這裡並非反對運動,或是說運動沒有效,而是希望大家把觀念著重在過"好動"的生活,也就是增加一般的生理活動量,而非過度執著於找時間去做運動,結果流於沒有時間,僅止於”想”的程度。 提升生理活動的方法 如果真的不容易養成運動的習慣(可能是沒時間,缺乏人陪無法持續或其他),你可以選擇養成好動的習慣,例如: 1、以騎單車來取代騎機車或開車。 2、用走路去買三餐或買東西。 3、 養成固定的打掃習慣。 4、用爬樓梯來取代電梯。 5、 提早一至兩站下車或將車停遠一點,增加走路活動的機會。 6、多幫忙做家事或幫忙蹓狗等。 7、 飯後到學校操場,公園綠地或堤防等地方散散步。 8、 假日多從事好動的活動,如登山露營,踏青郊遊,逛家壓馬路。 9、找運動夥伴,固定從事運動(如打球,游泳等)。 記得,勿以”動少”而不為,積少成多這是提升生理活動的座右銘。 【三、食物攝食產熱效應】 所謂的食物攝食產熱效應是指食物攝食過程中消化吸收等過程所消耗的能量,基本上,佔新陳代謝的10%左右。吃越精緻的食物所需要用來消化的熱量越少,吃越粗糙的食物,用來消化吸收所需的熱量越多。 提升食物攝食產熱效應的方法 1、減少油脂的攝取(少吃油炸或油膩的食物)。每吃100大卡的油脂有97大卡會轉變為脂肪儲存,所消耗的熱量最少。 2、以胚芽米、五穀飯或糙米飯來取代白米飯。 3、以瘦肉來取代絞肉。 4、多吃蔬菜水果,少吃餅乾等精緻加工的食物。 5、以新鮮水果來取代果汁。 6、不要喝含糖飲料或濃湯等較不需消化的食物。 正常落髮:平均每日掉髮約100-150根,落髮量與年紀有關。 (不用過度擔心) 1、家族史:是否有掉髮的家族遺傳史 2、抓髮測試:以手指抓取一小掫約25到30根,稍施力拉超過6根脫落為異常 3、圈髮測試:用拇指與食指將所有的頭髮往後圈成馬尾,直徑小於10元銅板 4、枕頭落髮量:平均每天落髮在枕頭上超過35根為異常 5、觀察髮根:髮根無透明毛囊內鞘,為異常角化現象 6、髮際線:前額左右兩側頭髮掉落,髮際線往後退 雄性禿可說是最悲慘的掉髮族群,因為命運的枷鎖讓自己逃都逃不掉。
有雄性禿基因者有高達1/3的機會會禿髮,原因在於雄性荷爾蒙如果代謝成二氫睪固酮,會讓毛囊萎縮,通常在 30多歲至40歲間濃度會很高,若壓力大或熬夜可能濃度會更高,造成落髮的情形就會更嚴重。 至於有家族史者會不會提早發生難定論,平時可以請自己的髮型師幫忙注意,尤其男生很少換髮型師,因此當髮型師覺得你的頭髮變少時就要去看診,另外,髮線愈來愈高,從眉毛往上8、9公分以上就得注意了。 有禿髮基因的人無論男女,總會在某個點,例如壓力大、睡眠不足、飲食不正常後,突然開始大量掉髮,就像掀開了潘朵拉的盒子一發不可收拾。 簡銘成醫師提醒民眾有三大徵兆要注意。 第一型:區塊(島嶼狀分佈)掉髮: 有些區塊(島嶼狀分佈)沒有頭髮,無論是圓型禿、黴菌感染或瘢痕性掉髮,這些問題都是頭皮頭髮生病了得趕快就醫,找到原因後,只要毛囊沒有完全破壞,是有可能恢復的。 第二型:短期掉髮數量明顯增加: 是掉髮數量明顯增加,平常可能只有掉十幾根髮,突然一次掉2、30根,主要是頭髮大量進入休止期。簡銘成醫師說,這個徵兆頭皮可能沒事,但要找出原因得回顧病人的病史,例如生產後、開完刀或經歷重大事件後發生。時間是最好的解決方式,塗養髮液可以加速復原。 第三型:型態性掉髮,M型或是地中海型: 這是最常見的雄性禿或女性掉髮。這類患者掉髮是在「無形」中增加,簡銘成形容,就像溫水煮青蛙,一次增加十根掉髮,根本不會在意,等發現時恐怕已進入雄性禿第三期了。 最後提醒雄性禿的朋友,確診有雄性禿的情況,請勿自行斷藥,該口服藥或外用藥治療還是要遵照醫師藥師指示,正確使用藥品。 |
依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。
恕不對外開放非專業人士使用。 每月文章
一月 2023
類別 |
營業時間:週一至週日(全年無休) 早上九點至晚上十一點四十分 (09:00~23:40)
|
地址:台北市松山區饒河街204號
|
聯絡我們 |




 RSS 訂閱
RSS 訂閱
