|
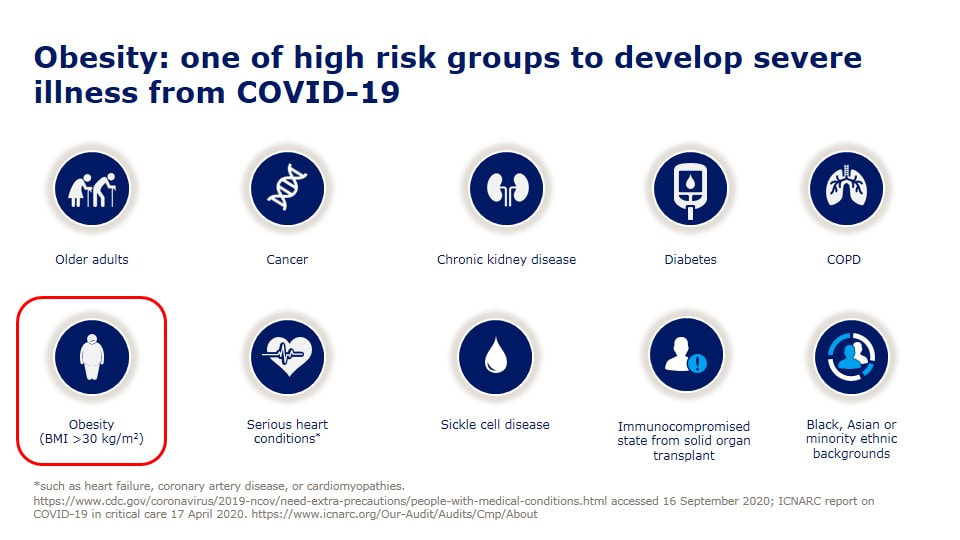
今天看到死亡個案出現一個「無慢性病史」的30幾歲女性,怎麼會這樣呢? 沒多久就就看到這個報導, 30多歲女「快速死」PCR陰轉陽5天不治 體重90公斤致病情惡化 「肥胖症」其實早就被世界衛生組織正名是一種「脂肪相關慢性疾病」(Adiposity-Based Chronic Disease (ABCD) )積極治療肥胖根本不是外觀的問題,而是跟治療高血壓、糖尿病一樣,刻不容緩的事情。 在今年四月前新冠死亡率居全球之冠的捷克,每10萬人就有229人染疫喪命,而捷克的肥胖是全歐洲之冠,女性57%肥胖、男性71%肥胖,10個新冠患者有8個肥胖, 新冠死亡率被認為跟肥胖有明確且嚴重的相關性。 台灣目前是亞洲的肥胖冠軍........ 一些區域的研究,發現糖尿病會讓新冠病毒死亡的風險多了8倍,而肥胖可以讓死亡風險高達10倍。 如果控制血糖跟血壓很重要,那控制體重難道不是更重要嗎? 研究證實,當肥胖者減少5%以上體重(如成人90公斤,減少5公斤),就可以為健康帶來許多益處,高血壓、糖尿病等與肥胖相關疾病將可改善。 強烈建議以後新冠肺炎死亡病例的「慢性病史」,麻煩一定要把「肥胖」列入在裡面,才能讓民眾正視自己的健康問題! 對於醫療人員來說處理肥胖,也是非常緊急的事,當BMI超過25時,每增加1單位,相對所有原因死亡率風險便增加9%,包括心因性猝死風險,是的,先不提快樂缺氧了,每個BMI在30以上的人,我都會嚴正告訴他猝死的風險,千萬不要因為自己年輕、或是血液報告尚可,就忽視了隱藏的風險。 也可以參考美國疾病管制局 www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html OverviewAdults of any age with the following conditions can be more likely to get severely ill from COVID-19. Severe illness means that a person with COVID-19 may need:
Preventive measures for COVID-19 (including vaccination, wearing a mask and social distancing) are important especially if you are older or have multiple or severe health conditions. You can learn about CDC’s COVID-19 vaccine recommendations, including how medical conditions and other factors inform recommendations, here. Note: The list below does not include all potential medical conditions that could make you more likely to get severely ill. Rare medical conditions may not be included below. However, a person with a condition that is not listed may still be in more danger from COVID-19 than persons of similar age who do not have the condition and should talk with their healthcare provider. Top of PageMedical Conditions in Adults
Get more information:
Get more information: Chronic lung diseases, including COPD (chronic obstructive pulmonary disease), asthma (moderate-to-severe), interstitial lung disease, cystic fibrosis, and pulmonary hypertensionChronic lung diseases can make you more likely to get severely ill from COVID-19. These diseases may include:
Get more information: Diabetes (type 1 or type 2)Having either type 1 or type 2 diabetes can make you more likely to get severely ill from COVID-19. Get more information:
Get more information: Heart conditions (such as heart failure, coronary artery disease, cardiomyopathies or hypertension)Having heart conditions such as heart failure, coronary artery disease, cardiomyopathies, and possibly high blood pressure (hypertension) can make you more likely to get severely ill from COVID-19. Get more information: HIV infectionHaving HIV (Human Immunodeficiency Virus) can make you more likely to get severely ill from COVID-19. Get more information: Immunocompromised state (weakened immune system)Having a weakened immune system can make you more likely to get severely ill from COVID-19. Many conditions and treatments can cause a person to be immunocompromised or have a weakened immune system. Primary immunodeficiency is caused by genetic defects that can be inherited. Prolonged use of corticosteroids or other immune weakening medicines can lead to secondary or acquired immunodeficiency. Get more information:
Get more information:
Get more information:
Get more information:
Get more information: Smoking, current or formerBeing a current or former cigarette smoker can make you more likely to get severely ill from COVID-19. If you currently smoke, quit. If you used to smoke, don’t start again. If you’ve never smoked, don’t start. Get more information:
Get more information: Stroke or cerebrovascular disease, which affects blood flow to the brainHaving cerebrovascular disease, such as having a stroke, can make you more likely to get severely ill from COVID-19. Get more information: Substance use disordersHaving a substance use disorder (such as alcohol, opioid, or cocaine use disorder) can make you more likely to get severely ill from COVID-19. Get more information:
Actions You Can TakeIn general, the older you are, the more health conditions you have, and the more severe the conditions, the more important it is to take preventive measures for COVID-19 such as vaccination, wearing a mask , social distancing, and practicing hand hygiene. Please contact your state, tribal, local, or territorial health department for more information on COVID-19 vaccination in your area. It is important for people with medical conditions and their providers to work together and manage those conditions carefully and safely. Get a COVID-19 vaccine as soon as you can. If you have a medical condition, the following are actions you can take based on your medical conditions and other risk factors:
0 評論
美國食品及藥物管理局(FDA)7日通過首款有助於減緩阿茲海默症(Alzheimer's disease)認知退化的藥物。研究證明,美國藥廠百健(Biogen)研發、名稱為Aducanumab的新藥,可以讓阿茲海默症引起的輕度認知障礙及早期失智症(dementia),雙雙獲得改善。根據估計,每名阿茲海默症患者使用Aducanumab,一年下來估計花費為5萬元。 華盛頓郵報指出,這是第一款證實可以減緩腦部功能退化而獲得食藥局通過的新藥,不只是僅僅能夠舒緩症狀而已。從2003年以來,並沒有任何阿茲海默症獲得食藥局通過,由此可見阿茲海默症藥物研發失敗率極高。 Aducanumab新藥研發過程中,曾引發正反兩派論戰。支持者認為,這款藥品獲得食藥局核可之後,可望引來各界對於阿茲海默症更高關注,進而帶動對於治療阿茲海默症解方的更多研究與投資。 百健藥廠表示,Aducanumab的問世能讓阿茲海默症患者爭取到更多寶貴時間與家人相處,患者的日常生活也可以自理,例如清潔、購物等。 Aducanumab是實驗室研發的蛋白質「單株抗體」(monoclonal antibody),必須透過靜脈注射,啟動患者體內的反疫反應,清除腦部澱粉蛋白斑塊。百健藥廠強調,這款藥品並不會治好阿茲海默症。 然而,反對派人士則表示,Aducanumab藥品是否確實有效,數據資料薄弱,食藥局為這款新藥放行,反映出主管機關迫於病患及倡議團體壓力之下,降低審查標準。 「阿茲海默症協會」(Alzheimer's Association)指出,美國目前約有620萬名阿茲海默症患者,如果沒有突破療法出現,患者人數到了2050年可望翻倍。 文章來源:www.worldjournal.com/wj/story/121469/5516439 FDA’s Decision to Approve New Treatment for Alzheimer’s Disease By Dr. Patrizia Cavazzoni, Director, FDA Center for Drug Evaluation and Research
Today FDA approved Aduhelm (aducanumab) to treat patients with Alzheimer’s disease using the Accelerated Approval pathway, under which the FDA approves a drug for a serious or life-threatening illness that may provide meaningful therapeutic benefit over existing treatments when the drug is shown to have an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients and there remains some uncertainty about the drug’s clinical benefit. This approval is significant in many ways. Aduhelm is the first novel therapy approved for Alzheimer’s disease since 2003. Perhaps more significantly, Aduhelm is the first treatment directed at the underlying pathophysiology of Alzheimer’s disease, the presence of amyloid beta plaques in the brain. The clinical trials for Aduhelm were the first to show that a reduction in these plaques—a hallmark finding in the brain of patients with Alzheimer’s—is expected to lead to a reduction in the clinical decline of this devastating form of dementia. We are well-aware of the attention surrounding this approval. We understand that Aduhelm has garnered the attention of the press, the Alzheimer’s patient community, our elected officials, and other interested stakeholders. With a treatment for a serious, life-threatening disease in the balance, it makes sense that so many people were following the outcome of this review. Further, the data included in the applicant’s submission were highly complex and left residual uncertainties regarding clinical benefit. There has been considerable public debate on whether Aduhelm should be approved. As is often the case when it comes to interpreting scientific data, the expert community has offered differing perspectives. At the end of the day, we followed our usual course of action when making regulatory decisions in situations where the data are not straightforward. We examined the clinical trial findings with a fine-tooth comb, we solicited input from the Peripheral and Central Nervous System Drugs Advisory Committee, we listened to the perspectives of the patient community, and we reviewed all relevant data. We ultimately decided to use the Accelerated Approval pathway—a pathway intended to provide earlier access to potentially valuable therapies for patients with serious diseases where there is an unmet need, and where there is an expectation of clinical benefit despite some residual uncertainty regarding that benefit. In determining that the application met the requirements for Accelerated Approval, the Agency concluded that the benefits of Aduhelm for patients with Alzheimer’s disease outweighed the risks of the therapy. What the Data Show The late-stage development program for Aduhelm consisted of two phase 3 clinical trials. One study met the primary endpoint, showing reduction in clinical decline. The second trial did not meet the primary endpoint. In all studies in which it was evaluated, however, Aduhelm consistently and very convincingly reduced the level of amyloid plaques in the brain in a dose- and time-dependent fashion. It is expected that the reduction in amyloid plaque will result in a reduction in clinical decline. We know that the Peripheral and Central Nervous System Drugs Advisory Committee, which convened in November 2020 to review the clinical trial data and discuss the evidence supporting the Aduhelm application, did not agree that it was reasonable to consider the clinical benefit of the one successful trial as the primary evidence supporting approval. The option of Accelerated Approval was not discussed by the Advisory Committee. As mentioned above, treatment with Aduhelm was clearly shown in all trials to substantially reduce amyloid beta plaques. This reduction in plaques is reasonably likely to result in clinical benefit. After the Advisory Committee provided its feedback, our review and deliberations continued, and we decided that the evidence presented in the Aduhelm application met the standard for Accelerated Approval. We thank the Advisory Committee for its independent review of the data and valuable advice. Accelerated Approval The FDA instituted its Accelerated Approval Program to allow for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need. Approval is based on a surrogate or intermediate clinical endpoint (in this case reduction of amyloid plaque in the brain). A surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. The use of a surrogate endpoint can considerably shorten the time required prior to receiving FDA approval. Drug companies are required to conduct post-approval studies to verify the anticipated clinical benefit. These studies are known as phase 4 confirmatory trials. If the confirmatory trial does not verify the drug’s anticipated clinical benefit, FDA has regulatory procedures in place that could lead to removing the drug from the market. The Devastation of Alzheimer’s Disease With all this said, we are extremely aware of the gradual and cumulative devastation that Alzheimer’s disease causes, as patients lose their memory and cognitive functioning over time. In late-stage disease, people can no longer hold a conversation or respond to their environment. On average, a person with Alzheimer’s disease lives four to eight years after diagnosis, but some patients can live up to 20 years with the disease. The need for treatments is urgent: right now, more than 6 million Americans are living with Alzheimer’s disease and this number is expected to grow as the population ages. Alzheimer's is the sixth leading cause of death in the United States. Although the Aduhelm data are complicated with respect to its clinical benefits, FDA has determined that there is substantial evidence that Aduhelm reduces amyloid beta plaques in the brain and that the reduction in these plaques is reasonably likely to predict important benefits to patients. As a result of FDA’s approval of Aduhelm, patients with Alzheimer’s disease have an important and critical new treatment to help combat this disease. FDA will continue to monitor Aduhelm as it reaches the market and ultimately the patient’s bedside. Additionally, FDA is requiring Biogen to conduct a post-approval clinical trial to verify the drug’s clinical benefit. If the drug does not work as intended, we can take steps to remove it from the market. But hopefully, we will see further evidence of benefit in the clinical trial and as greater numbers of people receive Aduhelm. As an agency, we will also continue to work to foster drug development for this catastrophic disease. 文章來源:www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease?fbclid=IwAR2_dfZqAgfSkGGHOcLFtrZvjsTYuDvhB9kymPQr7C3y4pzi9vBLzw_XgRA 「新英格蘭醫學」(New England Journal of Medicine)網路期刊和美國臨床腫瘤醫學會(American Society of Clinical Oncology)發表的一項跨國研究發現,英國藥廠阿斯特捷利康公司 (AstraZeneca PLC) 與美國藥廠默克公司 (Merck & Co.) 銷售的藥物Lynparza,可減少早期具侵襲性乳癌女性患者的癌症復發機率。 這項長期研究是遺傳性癌症治療的最新發展,為人類對抗遺傳性乳癌增添生力軍;也證明製藥業花在新型藥物PARP抑製劑方面的昂貴投資,是值得的。 Lynparza藥在美國定價為每位患者每月1萬4449元,是阿斯特捷利康公司最暢銷產品之一,主要用於治療晚期BRCA基因突變乳癌;去年銷售額達18億元。其競爭對手葛蘭素史克(GlaxoSmithKline)2019年斥資逾50億元收購另一款PARP抑製劑Tesaro製藥廠。 阿斯特捷利康癌症部門執行副總裁弗雷德里克森(David Fredrickson)表示,阿斯特捷利康將把研究數據提交監管機構,請求批准將Lynparza用於早期BRCA基因突變乳癌治療。 PARP抑製劑的作用是截斷癌細胞修復自身DNA能力,並導致癌細胞死亡。近年來,衛生監管機構已批准此類藥物用於治療卵巢癌、乳癌、前列腺癌和胰腺癌。目前發現這些藥物對BRCA1和BRCA2基因突變癌症,特別有用。 有BRCA基因突變的女性,罹患乳癌風險更高且通常更年輕。基因突變約占美國每年確診28萬1000椿乳癌病例的5%。根據美國癌症協會數據,乳癌是女性罹癌死亡第二大原因,每年在美國造成約4萬3600人死亡。美國食品藥物管理局(FDA)已在2018年批准使用Lynparza治療晚期的BRCA基因突變乳癌。 治療3年 近86%未復發 此項研究從2014年開始,在美國和其他22個國家/地區有1836名罹患早期BRCA1或 BRCA2乳癌的女性,在參加研究前均接受過切除腫瘤手術,並在手術前後接受防止腫瘤復發的化療。根據腫瘤大小或存在於淋巴結的癌症情況,她們的復發風險都很高。 研究隨機分配一半女性每天服用Lynparza藥劑一年,另一半服用安慰劑;研究人員發現,治療開始後2年半的中位隨訪期內,與安慰劑相比,Lynparza降低了42%癌症復發或任何原因死亡的綜合風險。治療三年後,接受Lynparza治療的女性85.9%沒有復發,接受安慰劑的女性77.1%未復發。 以上文章內容摘自世界新聞網。www.worldjournal.com/wj/story/121187/5511226 Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer Abstract
BACKGROUND Poly(adenosine diphosphate–ribose) polymerase inhibitors target cancers with defects in homologous recombination repair by synthetic lethality. New therapies are needed to reduce recurrence in patients with BRCA1 or BRCA2 germline mutation–associated early breast cancer. METHODS We conducted a phase 3, double-blind, randomized trial involving patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer with BRCA1 or BRCA2 germline pathogenic or likely pathogenic variants and high-risk clinicopathological factors who had received local treatment and neoadjuvant or adjuvant chemotherapy. Patients were randomly assigned (in a 1:1 ratio) to 1 year of oral olaparib or placebo. The primary end point was invasive disease–free survival. RESULTS A total of 1836 patients underwent randomization. At a prespecified event-driven interim analysis with a median follow-up of 2.5 years, the 3-year invasive disease–free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% confidence interval [CI], 4.5 to 13.0; hazard ratio for invasive disease or death, 0.58; 99.5% CI, 0.41 to 0.82; P<0.001). The 3-year distant disease–free survival was 87.5% in the olaparib group and 80.4% in the placebo group (difference, 7.1 percentage points; 95% CI, 3.0 to 11.1; hazard ratio for distant disease or death, 0.57; 99.5% CI, 0.39 to 0.83; P<0.001). Olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (hazard ratio, 0.68; 99% CI, 0.44 to 1.05; P=0.02); however, the between-group difference was not significant at an interim-analysis boundary of a P value of less than 0.01. Safety data were consistent with known side effects of olaparib, with no excess serious adverse events or adverse events of special interest. CONCLUSIONS Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer survival free of invasive or distant disease than was placebo. Olaparib had limited effects on global patient-reported quality of life. (Funded by the National Cancer Institute and AstraZeneca; OlympiA ClinicalTrials.gov number, NCT02032823. opens in new tab.) 原文出處:NEJM(www.nejm.org/doi/full/10.1056/NEJMoa21052155) 美國聯邦食品暨藥物管理局 (FDA) 4日核准一款糖尿病藥物Wegovy在美國販售,稱此藥可協助肥胖症患者進行長期減重;根據該藥物研究報告指出,使用Wegovy的試驗者平均減重15%,大約是34磅(15.3公斤)。 研究報告顯示,服用該藥物的受測者可在14個月內維持體重下滑,直到體重變化趨於穩定;至於服用安慰劑的對照組,受測者體重平均只減少2.5%,不超過六磅。 協助該藥物研究報告、路易維爾代謝與動脈粥狀硬化研究中心(Louisville Metabolic and Atherosclerosis Research Center)醫學主任貝斯(Harold Bays)表示:「市面上目前有的藥物只能幫助減重大約5%至10%,有時候甚至不到。」 全美罹患肥胖症的成人人口超過1億人,占比約三分之一;一般人若減重5%就能大幅改善行動力、高血壓、高血糖和膽固醇指數等健康問題;但貝斯表示,對肥胖症的患者來說,只減重5%是不夠的。 貝斯強調,比起一些初期用於治療肥胖症的藥物,Wegovy更加安全;Wegovy最常見的副作用包括腸胃不適、噁心、腹瀉和嘔吐,但這些症狀都會慢慢消退,但仍有5%的試驗者最終停止使用。 Wegovy對罹患特定甲狀腺瘤的患者具有潛在風險,因此不建議家族有甲狀腺瘤或內分泌瘤病史的人使用;此外,該藥物也會增加憂鬱症和胰臟發炎的風險。 Wegovy由丹麥藥廠諾和諾德(Novo Nordisk)研發,是一種用來抑制食慾的腸道激素,比另一款糖尿病用藥semaglutide的劑量還高;糖尿病患者每周使用一次Wegovy,並搭配飲食調整和運動達到減重的效果。 諾和諾德還未公布Wegovy的定價,但據稱和另一款減肥藥Saxenda的價格不相上下;Saxenda為每日注射,如果使用者沒有保險,一個月的費用在1300美元以上。(約39000元新台幣) 休士頓衛理公會醫院集團(Houston Methodist Hospitals)糖尿病首席醫師薩度(Archana Sadhu)表示,Wegovy的成效將取決於定價,因為有時候健保並不給付減肥藥物,定價太高恐怕使需要的人無法獲得。 以上文章內容摘自世界新聞網。www.worldjournal.com/wj/story/121617/5512354 提醒大家,此藥品在台灣尚未通過TFDA核准。 以下是美國FDA發表的新聞稿: (該新聞稿網址: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014) FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014 Today, the U.S. Food and Drug Administration approved Wegovy (semaglutide) injection (2.4 mg once weekly) for chronic weight management in adults with obesity or overweight with at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol), for use in addition to a reduced calorie diet and increased physical activity. This under-the-skin injection is the first approved drug for chronic weight management in adults with general obesity or overweight since 2014. The drug is indicated for chronic weight management in patients with a body mass index (BMI) of 27 kg/m2 or greater who have at least one weight-related ailment or in patients with a BMI of 30 kg/m2 or greater. “Today’s approval offers adults with obesity or overweight a beneficial new treatment option to incorporate into a weight management program,” said John Sharretts, M.D., deputy director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “FDA remains committed to facilitating the development and approval of additional safe and effective therapies for adults with obesity or overweight.” Approximately 70% of American adults have obesity or overweight. Having obesity or overweight is a serious health issue associated with some leading causes of death, including heart disease, stroke and diabetes, and is linked to an increased risk of certain types of cancer. Losing 5% to 10% of body weight through diet and exercise has been associated with a reduced risk of cardiovascular disease in adult patients with obesity or overweight. Wegovy works by mimicking a hormone called glucagon-like peptide-1 (GLP-1) that targets areas of the brain that regulate appetite and food intake. The medication dose must be increased gradually over 16 to 20 weeks to 2.4 mg once weekly to reduce gastrointestinal side effects. Wegovy should not be used in combination with other semaglutide-containing products, other GLP-1 receptor agonists, or other products intended for weight loss, including prescription drugs, over-the-counter drugs, or herbal products. Wegovy has not been studied in patients with a history of pancreatitis. Wegovy’s safety and efficacy were studied in four 68-week trials. Three were randomized, double-blind, placebo-controlled trials (including 16 weeks of dose increases) and one was a double-blind, placebo-controlled, randomized withdrawal trial in which patients receiving Wegovy either continued with the treatment or switched to a placebo. More than 2,600 patients received Wegovy for up to 68 weeks in these four studies and more than 1,500 patients received placebo. The largest placebo-controlled trial enrolled adults without diabetes. The average age at the start of the trial was 46 years and 74% of patients were female. The average body weight was 231 pounds (105 kg) and average BMI was 38 kg/m2. Individuals who received Wegovy lost an average of 12.4% of their initial body weight compared to individuals who received placebo. Another trial enrolled adults with type 2 diabetes. The average age was 55 years and 51% were female. The average body weight was 220 pounds (100 kg) and average BMI was 36 kg/m2. In this trial, individuals who received Wegovy lost 6.2% of their initial body weight compared to those who received placebo. The most common side effects of Wegovy include nausea, diarrhea, vomiting, constipation, abdominal (stomach) pain, headache, fatigue, dyspepsia (indigestion), dizziness, abdominal distension, eructation (belching), hypoglycemia (low blood sugar) in patients with type 2 diabetes, flatulence (gas buildup), gastroenteritis (an intestinal infection) and gastroesophageal reflux disease (a type of digestive disorder). The prescribing information for Wegovy contains a boxed warning to inform healthcare professionals and patients about the potential risk of thyroid C-cell tumors. Wegovy should not be used in patients with a personal or family history of medullary thyroid carcinoma or in patients with a rare condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Wegovy should not be used in patients with a history of severe allergic reactions to semaglutide or any of the other components of Wegovy. Patients should stop Wegovy immediately and seek medical help if a severe allergic reaction is suspected. Wegovy also contains warnings for inflammation of the pancreas (pancreatitis), gallbladder problems (including gallstones), low blood sugar, acute kidney injury, diabetic retinopathy (damage to the eye's retina), increased heart rate and suicidal behavior or thinking. Patients should discuss with their healthcare professional if they have symptoms of pancreatitis or gallstones. If Wegovy is used with insulin or a substance that causes insulin secretion, patients should speak to their health care provider about potentially lowering the dose of insulin or the insulin-inducing drug to reduce the risk of low blood sugar. Healthcare providers should monitor patients with kidney disease, diabetic retinopathy and depression or suicidal behaviors or thoughts. The FDA granted the approval to Novo Nordisk. Semaglutide 1 mg injection (Ozempic) was first approved as a treatment for type 2 diabetes in 2017. Related Information NIH: Overweight & Obesity Statistics ### The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products. 有關該藥品的詳細說明,僅供專業醫療人員閱覽,恕不對外開放。 此文合乎著作權法第50條規定:「以中央或地方機關或公法人之名義公開發表之著作,在合理範圍內,得重製、公開播送或公開傳輸。」
|
依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。
恕不對外開放非專業人士使用。 每月文章
一月 2023
類別 |
營業時間:週一至週日(全年無休) 早上九點至晚上十一點四十分 (09:00~23:40)
|
地址:台北市松山區饒河街204號
|
聯絡我們 |

 RSS 訂閱
RSS 訂閱
