|
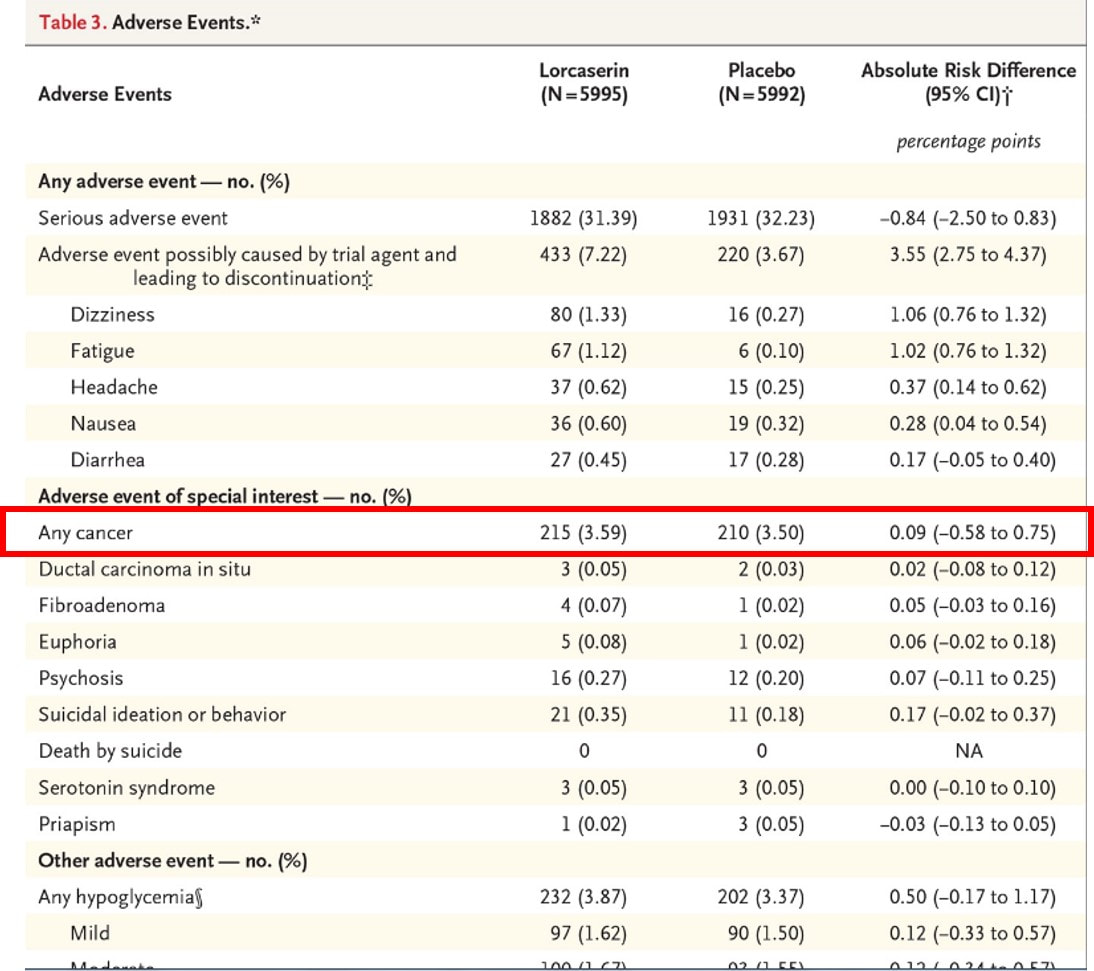
如果對詳細資料不感興趣的,可以直接看結論。 Drug Safety Communication - Due to Possible Increased Risk of Cancer 美國 FDA 在2020年1月14日發佈藥品安全警訊,大型隨機分派研究結果顯示,可能增加癌症風險!! 根據臨床的報告,受試者在3.3年的隨機試驗中,服用沛麗婷和安慰劑治療的患者,在新血管風險上無明顯差異,。 根據醫學博士Erin Bohula博士(馬薩諸塞州波士頓的布萊根婦女醫院)的介紹,在2018年歐洲心髒病學會大會上發表了試驗結果,當時沒有發現會增加致癌風險或加重精神疾病的副作用。 但在對該試驗進行了5年的後續訪查中,結果發現服用沛麗婷治療的患者中,罹患癌症的比例有所增加, 因此美國FDA在官網中發表該聲明:持續追蹤上市後的各項臨床數據。 也就是說,目前只是懷疑,FDA都尚未下定論,目前只是就上市後持續追蹤,來做一個提醒與警示。 每個藥品上市前後,都會持續追蹤各項臨床研究,癌症也是其中的一環,目前不必過度擔心。 至於在上市前的第三期臨床試驗:沒有增加罹癌風險的報告 在上市後的臨床研究(PMS):也沒有離癌風險的報告 而且FDA並沒有更進一步要求致癌風險的臨床研究。 加上根據臨床收錄的12000人中,都是年紀較大(平均年齡64歲),且肥胖(平均BMI=35,體重102公斤) 而且這個CVOT:Camellia Trial報告中,沛麗婷組只比安慰劑組多5例。在統計顯著性的實質意義,因此,不宜過度解讀。(215 V.S. 210) A Study to Evaluate the Effect of Long-term Treatment With BELVIQ (Lorcaserin HCl) on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors (CAMELLIA-TIMI)
(https://www.clinicaltrials.gov/ct2/show/NCT02019264) Cardiovascular Safety of Lorcaserin in Overweight or Obese Patients (https://www.nejm.org/doi/full/10.1056/NEJMoa1808721) (https://www.nejm.org/doi/full/10.1056/NEJMoa1808721) 可以看出肥胖本身,就是罹癌風險的因子。 結論:在上市後,持續追蹤滿5年的研究中,在年紀較大(平均年齡64歲),且肥胖(平均BMI=35,體重102公斤)發現罹患癌症的比例有所增加,但是是否因沛麗婷導致癌症,FDA也不清楚,還有待FDA更進一步的消息。 未來,若有任何更新的任何資訊,將第一時間為大家來追蹤。 歡迎追蹤新德大藥局的FB或是官網,隨時為您更新最資訊。 目前有很多藥品都有被FDA警示過,降血糖藥exenatide (Byetta, Bydureon), liraglutide (Victoza), sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), saxagliptin (Onglyza, Kombiglyze XR), alogliptin (Nesina, Kazano, Oseni), and linagliptin (Tradjenta, Jentadueto)、GnRH agonists、治療骨質疏鬆的雙磷. 酸鹽類(Bisphosphonate)藥等。這些藥品,目前也都持續使用,而且尚未停止使用或下架,所以不用過度解讀。 (https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-investigating-reports-possible-increased-risk-pancreatitis-and-pre) 以下是美國FDA原文: [Posted 01/14/2020] AUDIENCE: Patient, Health Professional, Pharmacy ISSUE: The FDA is alerting the public that results from a clinical trial assessing safety show a possible increased risk of cancer with the weight management medicine Belviq, Belviq XR (lorcaserin). At this time, the cause of the cancer is uncertain, and we cannot conclude that lorcaserin contributes to the cancer risk. However, we wanted to make the public aware of this potential risk. We are continuing to evaluate the clinical trial results and will communicate our final conclusions and recommendations when we have completed our review. BACKGROUND: Lorcaserin is a prescription medicine approved by FDA in 2012 for use with a reduced-calorie diet and increased physical activity to help weight loss in adults who are obese or are overweight and have weight-related medical problems. Lorcaserin works by increasing feelings of fullness so that less food is eaten. It is available as a tablet (Belviq) and an extended-release tablet (Belviq XR). RECOMMENDATION: Health care professionals should consider if the benefits of taking lorcaserin are likely to exceed the potential risks when deciding whether to prescribe or continue patients on lorcaserin. Patients currently taking lorcaserin should talk to their health care professionals about the potential increased risk of cancer with use of lorcaserin. Health care professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
https://www.fda.gov/safety/medical-product-safety-information/belviq-belviq-xr-lorcaserin-drug-safety-communication-due-possible-increased-risk-cancer 以下是官方聲明
1 評論
李寶宗
1/16/2020 11:50:49 am
原來如此,感謝分享
回覆
發表回覆。 |
依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。
恕不對外開放非專業人士使用。 每月文章
一月 2023
類別 |
營業時間:週一至週日(全年無休) 早上九點至晚上十一點四十分 (09:00~23:40)
|
地址:台北市松山區饒河街204號
|
聯絡我們 |


 RSS 訂閱
RSS 訂閱
