|
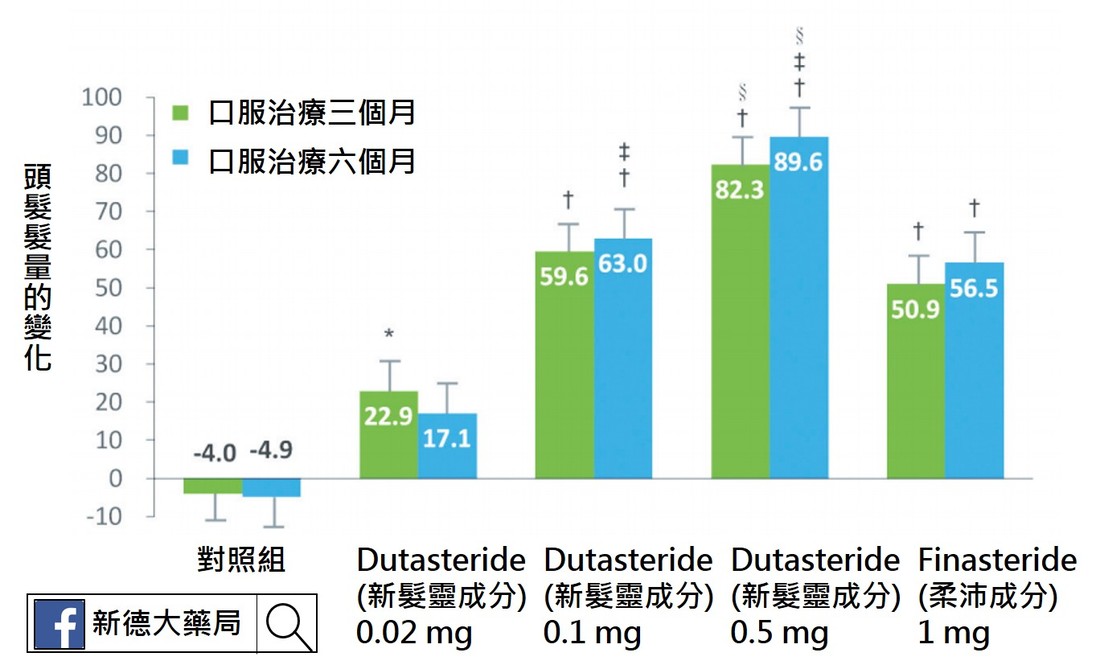
收錄917名雄性禿病人之跨國多中心試驗中,排除血中睪固酮濃度<250 ng/dL者,dutasteride(新髮靈)劑量為0.02、0.1或0.5mg,治療6個月後,dutasteride(新髮靈) 0.1 mg增加髮量和頭髮粗度之效果與Finasteride(柔沛) 1 mg相當,Dutasteride(新髮靈) 0.5mg增加髮量之效果較Finasteride(柔沛)高出30%。 Dutasteride(新髮靈)用於不同嚴重程度的受試者,皆有一定程度之療效。
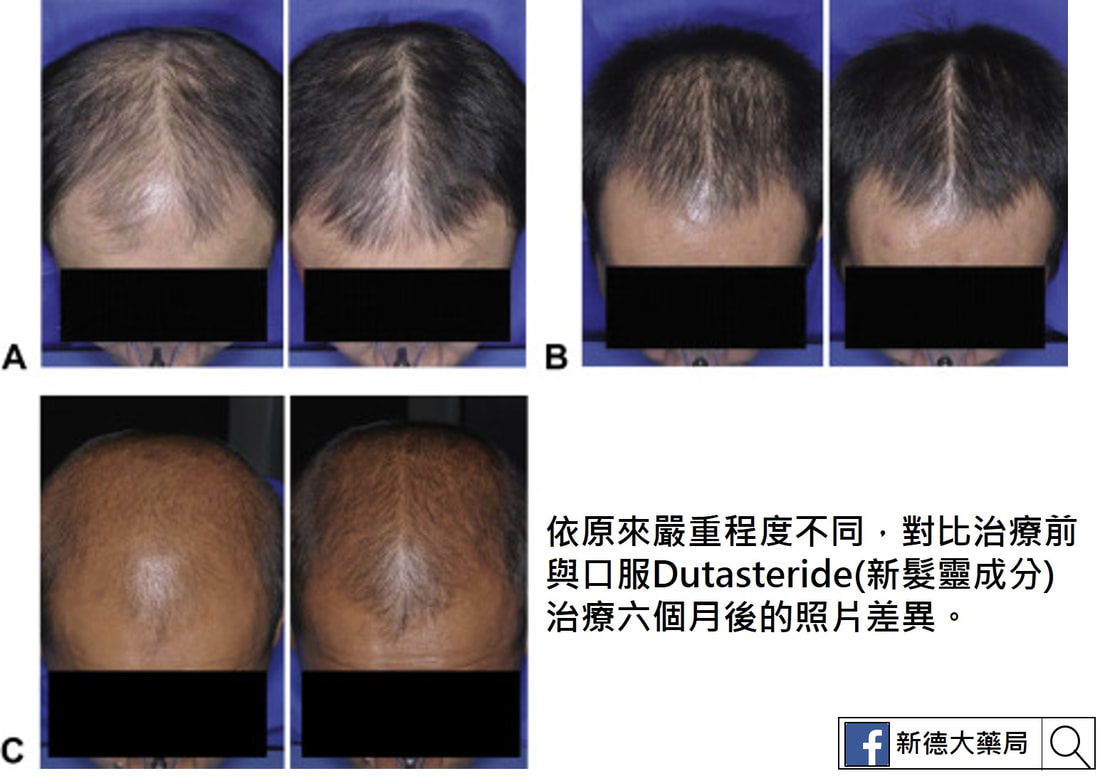
以Finasteride (柔沛)1 mg治療6個月後未達到顯著臨床效果的雄性禿病 人, 改以Dutasteride(新髮靈) 0.5 mg治療後,有77.4%達到程度不等之改善,於頭髮密度與粗度,平均改善了10.3%及19.8%。年輕的雄性禿病人經過24週治療後,使用dutasteride(新髮靈) 0.5 mg者單位面積髮量平均增加13根,使用Finasteride(柔沛) 1 mg者反而減少4根。 日本的研究以Dutasteride(新髮靈) 0.5 mg治療雄性禿病人,超過9成完成持續52週的治療,且頭頂和前額區域皆明顯改善,僅<1%受試者髮量減少。 參考文獻 1. Lee WS, Lee HJ, Choi GS, et al.: Guidel ines for management of androgenetic alopecia based on BASP classification–the Asian consensus committee guideline. JEur Acad Dermatol Venereol 2013; 27:1026-1034. 2. Eun HC, Kwon OS, Yeon JH, et al.: Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: A randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol 2010; 63:252-258. 3. Tsunemi Y, Irisawa R, Yoshiie H, et al.: Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J Dermatol 2016; 43:1051- 1058. 4. Marks LS: 5α-reductase: History and clinical importance. Rev Urol 2004; 6(suppl 9):S11-S21. 5. Blumeyer A, Tosti A, Messenger A, et al.: Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges 2011; Suppl 6:S1-S57. 6. Gupta M, Mysore V: Classifications of patterned hair loss: a review. J Cutan Aesthet Surg 2016; 9: 3-12. 7. Olsen EA, Dunlap FE, Funicella T, et al.: A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol 2002; 47:377-85. 8. Lucky AW, Piacquadio DJ, Ditre CM, et al.: A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol 2004; 50:541-53. 9. Hillmann K, Garcia Bartels N, Kottner J, et al.: A singlecentre, randomized, double-blind, placebo-controlled clinical trial to investigate the efficacy and safety of minoxidil topical foam in frontotemporal and vertex androgenetic alopecia in men. Skin Pharmacol Physiol 2015; 28:236- 244. 10. 蔡長祐、蔡仁雨:台灣男性雄性禿之診療現況,台灣醫界,2008;51:326-330。 11. Messenger AG, Rundegren J: Minoxidil: mechanisms of action on hair growth. Br J Dermatol 2004; 150:186-194. 12. Lulic Z, Inui S, Sim WY, et al.: Understanding patient and physician perceptions of male androgenetic alopecia treatments in Asia-Pacific and Latin America. J Dermatol 2017 Mar 31. doi: 10.1111/1346-8138.13832. [Epub ahead of print] 13. Roberts JL, Fiedler V, Imperato-McGinley J, et al.: Clinical dose ranging studies with finasteride, a type 2 5alphareductase inhibitor, in men with male pattern hair loss. J Am Acad Dermatol 1999; 41:555-563. 14. Olsen EA, Whiting DA, Savin R, et al.: Global photographic assessment of men aged 18 to 60 years with male pattern hair loss receiving finasteride 1 mg or placebo. J Am Acad Dermatol 2012; 67:379-386. 15. Kaufman KD, Rotonda J, Shah AK, et al.: Long-term treatment with finasteride 1 mg decreases the likelihood of developing further visible hair loss in men with androgenetic alopecia (male pattern hair loss). Eur J Dermatol 2008; 18:400-406. 16. Rossi A, Cantisani C, Scarnò M, et al.: Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther 2011; 24:455-461. 17. Olsen EA, Hordinsky M, Whiting D, et al.: The importance of dual 5a-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebocontrolled study of dutasteride versus finasteride. J Am Acad Dermatol 2006; 55:1014-1023. 18. Harcha WG, Martinez JB, Tsai TF, et al.: A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol 2014; 70:489-498. 19. Jung JY, Yeon JH, Choi JW, et al.: Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol 2014; 53:1351-1357. 20. Shanshanwal SJ, Dhurat RS: Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol 2017; 83:47-54.
0 評論
發表回覆。 |
依主管機關相關規定,專業醫藥資訊僅提供醫藥專業人員參考(請申請核可通過後,即可閱讀專業人員區)。
恕不對外開放非專業人士使用。 每月文章
一月 2023
類別 |
營業時間:週一至週日(全年無休) 早上九點至晚上十一點四十分 (09:00~23:40)
|
地址:台北市松山區饒河街204號
|
聯絡我們 |

 RSS 訂閱
RSS 訂閱
